Advance Research in Organic and Inorganic Chemistry
[ ISSN : 2833-3594 ]
pH Value of Blood: Should It be Considered as A Part of Standard Blood Biochemical Profile?
1Complementary and Integrative Health Clinic of Dr. Shishonin, 5 Yasnogorskaya Str, Moscow, 117588, Russia
2Institute of Biochemical Technology and Nanotechnology (IBTN) of the Peoples’ Friendship University of
Russia (RUDN), 6 Miklukho-Maklaya St, 117198 Moscow, Russia
Corresponding Authors
Keywords
Abstract
For over a century, physicians considered the pH value of blood as one of the paraments, changes that could alarm serious metabolism deviations. The medical community had developed two parallel approaches, traditional and Stewart, to evaluate acid-base phenomena. Nevertheless, such important parameter is still missing in a Standard Blood Biochemical Profile (SBBP), which is periodically required by Primary Care Physicians (PCP) to ensure the health status of their patients. The current review is devoted to the advantages, that the medical community could obtain after accepting blood pH values as general for analysis, the consideration of technical possibilities of test strips development for digital measurements, and some related issues. Particular interest is associated with the possible connection between the blood pH data and the validity of the theory of Centralized Aerobic-Anaerobic Energy Balance Compensation (CAAEBC).
Abbreviations
CAAEBC - centralized aerobic-anaerobic energy balance compensation NCD – non-communicable disease PCP – primary care physician(s) SBBP - standard blood biochemical profile WHO – World Health Organization
Introduction
The role of water in human life is hard to over appreciate, and namely for this reason WHO devotes so much attention to its quality, especially in its drinkable form [1]. Since 19th century experimentalists are working on the approaches, which give them an opportunity to find the content of water in the living human body or some associated with its parameters (e.g. daily water consumption) [2]. So far, the common view considers that during the lifetime the water content smoothly decreases from 75% at the very birth to 55% at death [3]. These numbers could be slightly different in different reports, but it is obvious that at least half of the body weight wise is simply water. It is getting obvious that the disturbances of water balance could cause a list of diseases [3], including even vestibular disorders [4]. Water possesses an intrinsic ability to dissociate into OH- and H+ (which exists in hydrated form H3 O+). It is worth reminding that the water dissociation constant is temperature-dependent. Shift of acid (H+)- base (OH- ) balance in the body can lead to molecular, cellular, and general health consequences. Therefore, the value of blood pH is clinically important not only to evaluate the physiological condition of critically ill patients but also to get general information about their health status. There are two approaches to characterize acid-base equilibrium in blood. Historically they are separated with three quarters of the century and reflect the growing understanding of blood ion balance complexity.
The first one historically, called traditional, based on the Henderson [5] -Hasselbalch [6] equation focuses on changes in the concentration of bicarbonate (HCO3-), the partial pressure of carbon dioxide (pCO2 ), the dissociation constant, and the solubility of CO2 . It should be noticed that temperature dependence of the CO2 solubility had a significant impact on temperature blood pH instability, which will be covered later. Development of this idea gave concepts of Base Excess (BE) [7] that smoothly grown up into base excess methodology [8,9]. Unfortunately, this approach experiences some complications in attempts to describe different types of metabolic acidosis [10]. The Anion Gap (AG), the difference between unmeasured plasma anions and the unmeasured plasma cations [11], is an additional diagnostic tool to assess the metabolic components of the acid–base equilibrium. Albumin and phosphate, one of the circulatory proteins, mainly account for the AG under normal conditions. This additional index provides some extras to the traditional approach, classifying metabolic acidosis into normal AG acidosis and high AG acidosis. However, severe pH disturbances and other factors demonstrate a significant influence on the AG [12, 13]. Those disadvantages lower the sensitivity and specificity of this diagnostic tool to detect metabolic acidosis. The acceptance of AG causes the necessity to introduce entire list of additional parameters:
- Strong Ion Difference (SID),
- Total concentration of weak acids (ATOT), and
- Partial pCO2 of the solution [11]
The Stewart’s approach changed the angle of consideration [14]. He considered ideas AG, SID, and ATOT [11] and modeled a blood as a complex mixture of ions of constant charge over the physiological pH range (strong ions), non-volatile proton donor/acceptors which transfer protons in the physiological pH range (weak acid/base), and the volatile bicarbonate–CO2 buffer system. In the Stewart’s approach, metabolic disorders appear to be the results of changes in SID or ATOT [15]. We need to underline, that there are some controversies over the evaluation of ATOT acidosis/alkalosis existence [16, 17], which should be left behind the frames of current report.
The idea of aquatic pH measurements with certain electrodes or indicators for chemists looks general, and physicians performed it in traditional way not only before [18] Stewart’s publication [14]. Namely then the initial studies were conducted, that reveal high instability of the blood pH right after the boosting it from blood vessel. But until 1984 even the issue of the manner of transportation of fresh blood samples was under the question because of the reported instability [19]. It means, that medical community was sure about importance of such data. Detailed studies revealed some impact on pH from syringe material [20], and other parameters [21] and so far, this problem has not been efficiently resolved [22].
Why pH measurements are so important for contemporary Medicine? Firstly, the maintaining of acid-base balance is considered by the modern fundamental Medicine as the condition to keep the patient in good health. If we visit Pubmed (https://pubmed.ncbi. nlm.nih.gov/) then “metabolic acidosis” gives over 56K references, “acid-base disorders” – over 5K, and “acid-base imbalance” – almost 41K. Moreover, the proposed recently CAAEBC theory directly connects certain NCDs with the changes in blood pH [23-25]. Below we will show the way from the dead-end where PCP who need pH data are trapped now. Nowadays, if they need to know blood pH value for their patients, the single solution is to send a patient to the hospital with the open surgery unit, where blood pH tests con be performed.
Discussion
It appears, that now each PCP, that needs blood pH data to diagnose his patient meets the issue of where to send the patient to measure it. It appears that the vast majority of the offices that are allowed to take blood samples outsource their processing to special labs. Since delivery time is long, the data is corrupted. Is it possible to ease the question – conduct the measurement just during the visit? The simplest answer is – YES. The simplest way is to employ over-the-counter glucometers is to employ the precision of data on glucose measurement, which would be close enough to laboratory venous plasma glucose measurements [26].
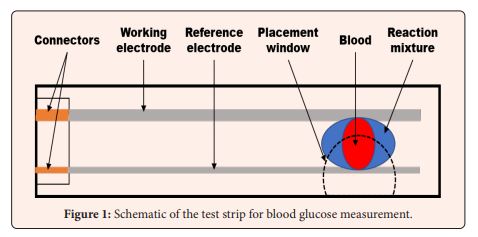
Glucometers? -Yes! Over-the-counter glucometers are devices that measure parameters of the electric signal from Red-Ox reaction at the moment when blood drop on the reaction mixture connects electrodes (Figure 1) with low-cost digital processing, since 1981 when Bayer introduced its Glucometer. Nowadays there are plenty of chemical solutions to the request of electric signal modulation depending on glucose concentration [27] and pH differences could be expressed in the same way in the form of differences in electric signals [28] from specially designed test-strips. It should be understandable that for such pH meters the market should be much wider than for glucometers. Unfortunately, contemporary idea to evaluate pH for the purpose of correct diagnosis appears to be much more popular among veterinarians, rather than PCPs [29].
Let’s underline:
- pH is important value for internal metabolism characterization and is essential index of acidosis characterization
- Metabolic acidosis is related to multiple diseases as chronic as well acute
- Currently blood pH measurements can be done only with very special devices in the very special labs (mostly in hospitals) and interpreted by well-trained professionals
- This paradigm should be changed for the sake of patients both in Medicine and Veterinary
- There are multiple possible chemical reactions that can be employed to generate electrical signal to measure pH in similar way to glucose in glucometer.
- The above-mentioned strips in case of development could be a valuable support to in house monitoring multiple NCDs.
Conclusion
We expect that we were able to convince the research community to reevaluate the convenience of simple blood pH measurements. It seems to be fruitful for both the patient and Primary Care Physician (or Veterinarian). We do not discuss what RedOx reaction from the list should be chosen, instead, we would like to challenge researchers to develop multiple technical solutions on the current level of technology development.
Funding
It has been supported by the RUDN University Strategic Academic Leadership Program (for AAV)
Acknowledgments
The authors wish to thank BS V.D Bystrykh for her assistance with the edition of the submission’s final version. Dr. Alexandre A. Vetcher expresses acknowledgments to the RUDN University Strategic Academic Leadership Program for the obtained support.
Conflict of Interest
The authors declare no conflict of interest.
References
3. Popkin BM, D'Anci KE, Rosenberg IH (2010) Water, hydration, and health. Nutr Rev 68(8): 439-458.
14. Stewart PA (1980) How to Understand Acid-Based: A Quantitative Acid-Base Primer for Biology and Medicine. Elsevier Science Ltd, New York,186p.
18. Straumfjord JV (1958) Standard Methods of Clinical Chemistry, Elsevier, Netherlands 2: 107-121.
Citation: Shishonin AY, Zhukov KV, Gasparuan BA and Vetcher AA (2022 pH Value of Blood: Should It be Considered as A Part of Standard Blood Biochemical Profile?. Adv Res Org Inorg Chem 3: 1012