Current Research in Emergency Medicine
[ ISSN : 2832-5699 ]
Acute Pericardial Effusion from Pacemaker Lead Perforation of Right Ventricle Identified on Bedside Emergency Department Ultrasonography
1Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, Florida, USA
2St. Mary’s Medical Center, West Palm Beach, Florida, USA
3Baptist Health Boca Raton Regional Hospital, Boca Raton, Florida, US
Corresponding Authors
Keywords
Abstract
Introduction and Objective
Cardiac pacemakers and Implantable Cardioverter-Defibrillators (ICD) are devices used to treat a range of cardiac dysrhythmias. Symptomatic cardiac perforation may occur in as many as 4.2% of pacemaker or ICD placements with 1.2% of pacemaker or ICD placements leading to clinically significant pericardial effusion. Acute onset of symptoms within 24 hours of device placement due to cardiac perforation is particularly rare, with a paucity of reported cases. This report describes a case of bedside Emergency Department (ED) ultrasound identifying an acute onset of pericardial effusion following cardiac pacemaker placement in an 85-year-old woman who experienced pleuritic and oscillating chest pain.
Case Presentation
An 85-year-old woman with a past medical history of hypertension, diabetes and dual-chamber pacemaker placement two days ago presented to the ED with pleuritic and oscillating chest pain. Initial bedside Point of Care Ultrasound (POCUS) exam, performed by the emergency department attending, revealed a moderate pericardial effusion with evidence of a pacemaker lead perforation through the right ventricular myocardium. Lead perforation and effusion were confirmed via formal transthoracic echocardiogram. Minutes later, the patient was treated with a right ventricle lead repositioning procedure and recovered uneventfully.
Conclusion
Symptomatic cardiac perforation due to pacemaker or ICD placement is rare, with a range of potential complications which typically occur weeks to months after placement. This case demonstrates the utility of POCUS exam for patients presenting to the ED for non-specific chest pain as it facilitated the timely diagnosis of an atypical presentation of ICD-induced wall perforation.
Introduction
Cardiac pacemakers and Implantable Cardioverter-Defibrillators (ICD) are devices used to treat a range of cardiac dysrhythmias [1]. Uncommonly, implantation can result in complications including but not limited to pneumothorax, pericarditis, pericardial effusion, pleural effusion and pulmonary embolism due to pacemaker dysfunction, lead misplacement or cardiac wall perforation [1-3]. Cardiac perforation following pacemaker or ICD placement is rare [3-7], however, it may be more common than previously suspected [2]. According to Hirschl et al. 2007, 15% of patients with cardiac pacemakers or ICDs may exhibit lead perforation, with a greater prevalence of perforation seen in the atria than the ventricles (15% vs 6%) and in ICD devices than pacemakers (14% vs 3%) [8].
Symptomatic cardiac perforation may occur in as many as 4.2% of pacemaker or ICD placements. Furthermore, 1.2% of pacemaker or ICD placements lead to clinically significant pericardial effusion [2,3]. Complications can occur within hours to months of device placement, with most reported cases presenting weeks to months after device placement [2,5,9]. Acute onset of symptoms within 24 hours of device placement due to cardiac perforation is particularly rare, with a paucity of reported cases [10]. Particularly in the acute setting, complications such as cardiac tamponade, hemopericardium and hemothorax can occur [6]. With these dangerous complications described in the acute setting, less severe complications following pacemaker or ICD placement are given modest clinical consideration in patients experiencing symptoms within 24 hours of implantation.
Herein, our case describes a case of bedside Emergency Department (ED) ultrasound identifying an acute onset of pericardial effusion following cardiac pacemaker placement in an 85-year-old woman who experienced pleuritic and oscillating chest pain.
Case Presentation
An 85-year-old woman, with a past medical history of hypertension, diabetes and dual-chamber pacemaker placement two days ago, presented to the ED on 3/19 with acute onset of severe chest pain. She woke up in the early morning around 3 am with a sudden onset of spasmodic chest pain recurring every ten minutes and lasting a few minutes each time. Upon examination, she reported intermittent oscillation of the pain from the left to right side of her chest, which occurred at rest and was accompanied by shortness of breath and fatigue.
The patient was observed overnight on 3/17 following pacemaker placement with no complications prior to hospital discharge. In the months before cardiac pacemaker placement, the patient experienced gradually increasing fatigue with an “abnormal” EKG two weeks before surgery. EKGs taken on 3/17 revealed complete heart block, with evidence of an age indeterminate anterior wall infarction, ventricular rate of 41 bpm, atrial rate of 75 bpm and corrected QT (QTc) interval of 448 milliseconds. During the two weeks between the discovery of this EKG and surgery, the patient experienced occasional intermittent chest discomfort, though she denied shortness of breath, nausea, diaphoresis, COVID-19 symptoms or fever. Her intermittent chest discomfort culminated in several episodes of sharp, left-sided chest pain at yoga, on the night of 3/16.
Upon hospital admission on 3/17, the patient was hemodynamically stable with a blood pressure of 162/60, regular rate and rhythm and a SpO2 of 95%. Laboratory results taken at this time are described in Table 1. An x-ray taken at this time found a normal heart size, normal mediastinal contour, and hypoventilatory changes/atelectasis of the left lower lobe, with no evidence of pericardial effusion or pneumothorax (Figure 1). The remainder of the hospital course was uneventful with symptom resolution and no further complaints per patient.
Table 1: Laboratory Results Taken Before and After Pacemaker Repositioning
|
Tests |
Laboratory Results on 3/17 |
Laboratory Results on 3/19 |
Normal Reference Values |
|
Pro-BNP (pg/mL) |
1167 |
1164 |
< 450 |
|
Troponin I (ng/mL) |
96 |
51 |
< 0.04 |
|
Glucose (Random) (mg/dL) |
123 |
154 |
< 140 (< 100 fasting) |
BNP: Brain Natriuretic Peptide; pg/mL: picograms/milliliter; ng/L: nanograms/liter; mg/dL: milligrams/deciliter
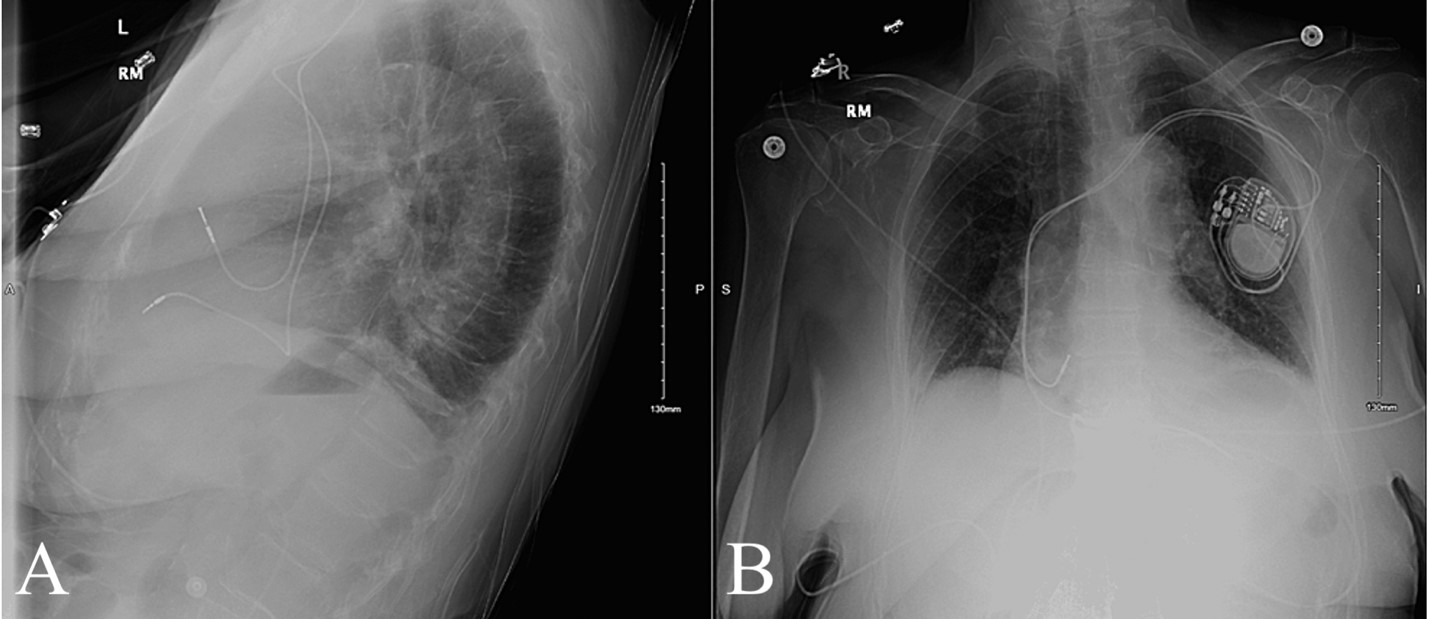 Figure 1: X-Ray Taken Following Pacemaker Implant .Figure 1A: Lateral View; Figure 1B: AP View
Figure 1: X-Ray Taken Following Pacemaker Implant .Figure 1A: Lateral View; Figure 1B: AP View
Examination revealed a regular heart rate, with normal S1 and S2, non-labored respirations with equal breath sounds, symmetrical chest wall expansion and clear auscultation bilaterally. Initial bedside point of care ultrasound (POCUS) exam, performed by the emergency department attending, revealed a moderate pericardial effusion with evidence of a pacemaker lead perforation through the right ventricular myocardium. Lead perforation and effusion were confirmed via formal Transthoracic Echocardiogram (TTE) (Figure 2).
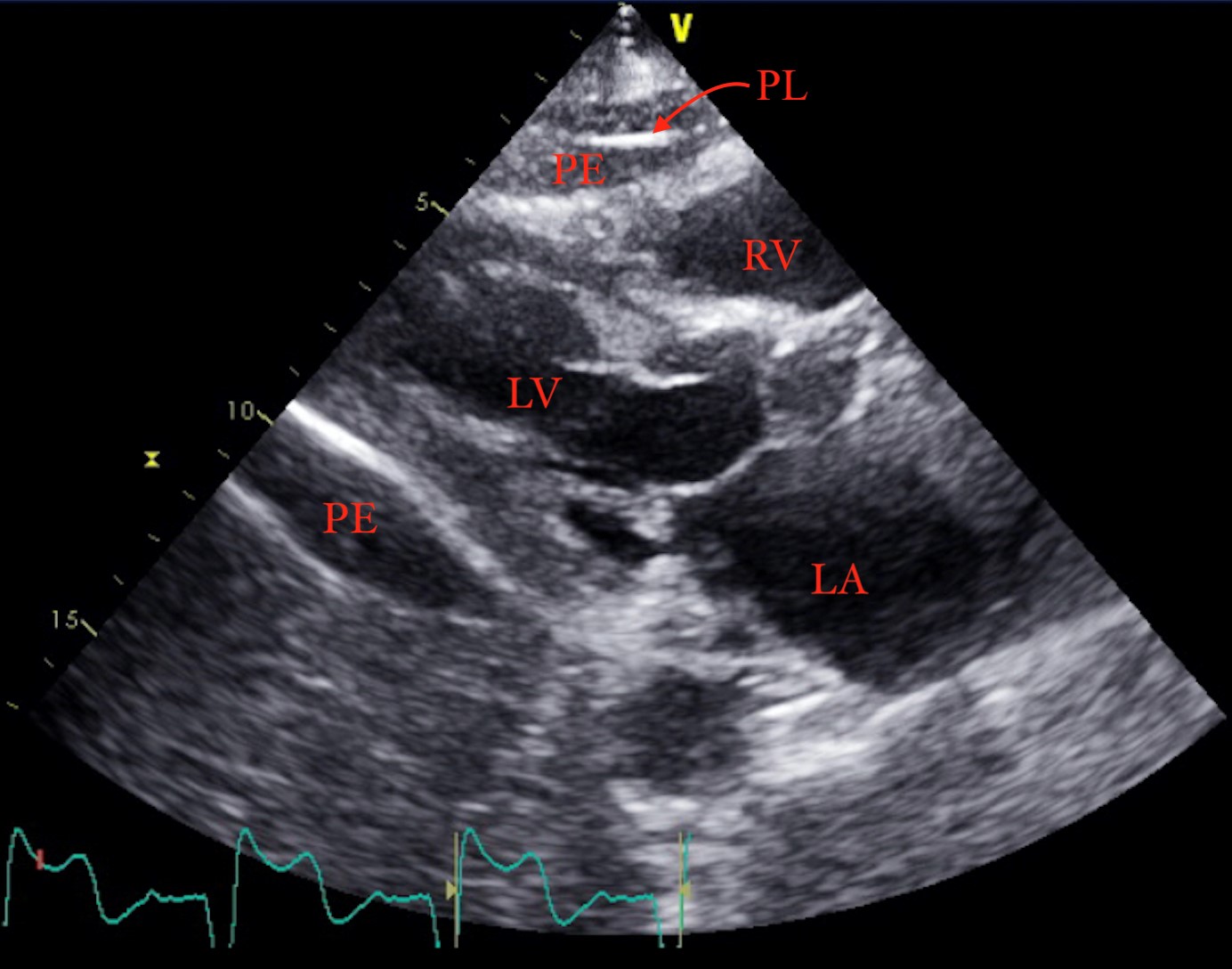
Figure 2: Pericardial Effusion on Transthoracic Echocardiogram
PL: Pacemaker Lead; PE: Pericardial Effusion; RV: Right Ventricle; LV: Left Ventricle; LA: Left Atrium
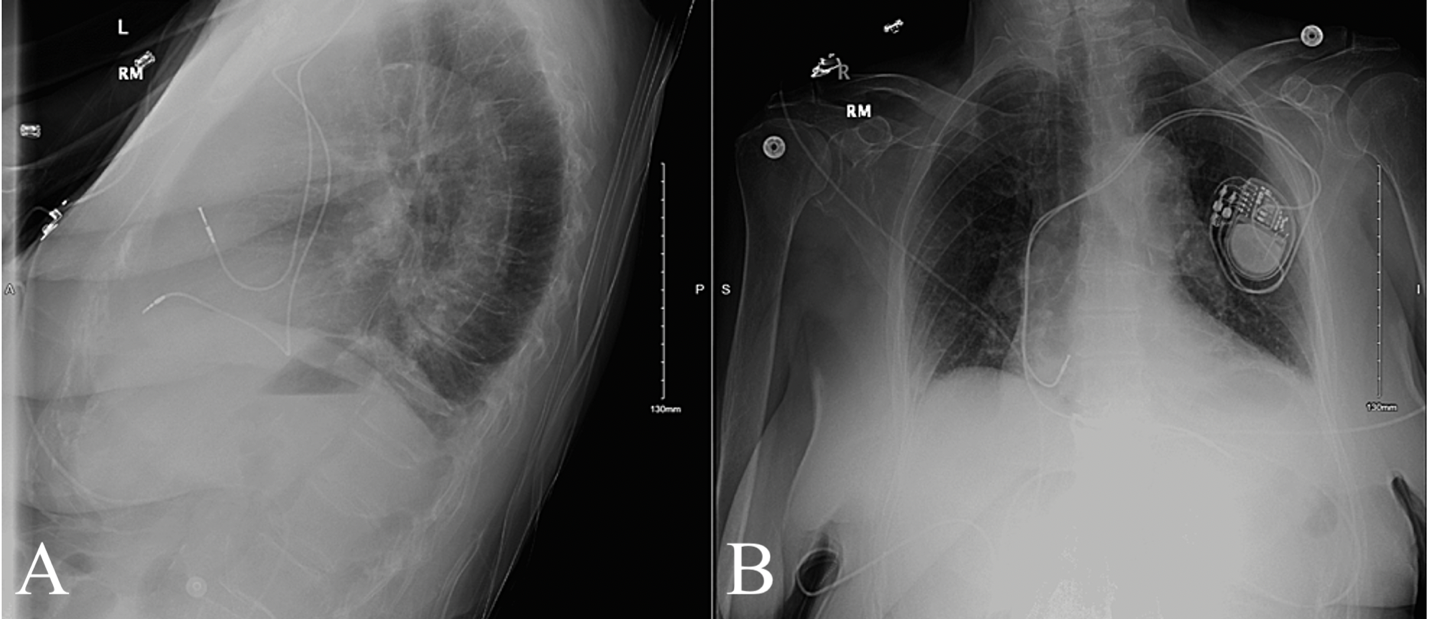
Within minutes, the patient was taken to the operating room with a cardiothoracic surgeon and electrophysiologist. The patient was treated with a Right Ventricle (RV) lead repositioning procedure. The tip and part of the lead body were pulled back through the myocardial perforation, from the pericardium, and repositioned into the base of the RV outflow tract. Repositioning occurred without complications and further imaging revealed stable placement of the repositioned lead (Figure 3). Patient recovery was uneventful, with symptom resolution and improvements in hemodynamic stability, mobility and subjective comfort. The patient was discharged two days later with evidence of trace pericardial effusion, normal vital signs and cardiovascular function.
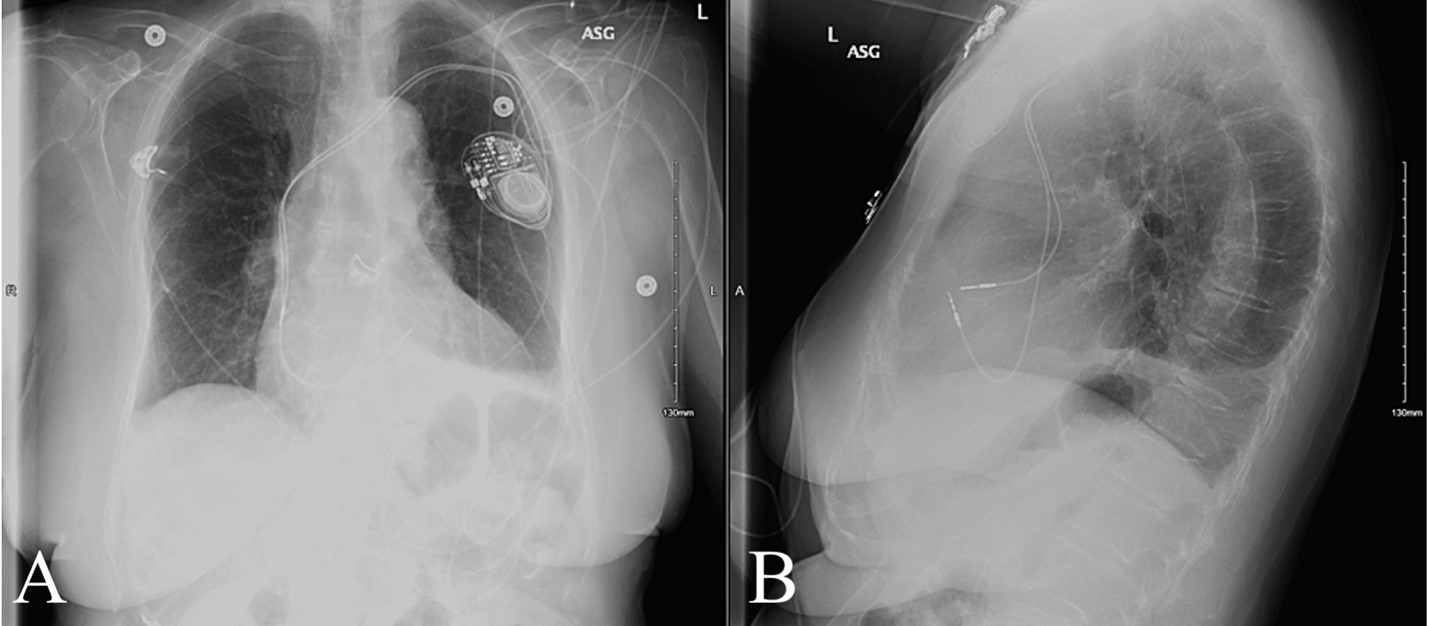 Figure 3: X-Ray Taken Following Pacemaker Repositioning Figure 3A: AP View; Figure 3B: Lateral View
Figure 3: X-Ray Taken Following Pacemaker Repositioning Figure 3A: AP View; Figure 3B: Lateral View
Discussion
This case demonstrates the importance of performing a POCUS exam and including cardiac wall perforation in the differential diagnosis of patients with chest pain, who have a pacemaker or ICD in place. Our patient is atypical in the acute onset of symptoms following pacemaker implantation, as well as the clinical presentation, which had features of myocardial infarction and pericardial effusion. Her presentation is unique among the small handful of past reported cases of cardiac implant-induced wall perforation presenting within 24 hours of implantation [4,6,7]. There are few reports of acute onset of cardiac implant-induced wall perforation.
However, past cases have reported severe consequences in the form of cardiac tamponade, hemopericardium and hemothorax [4,6]. This case presumes to establish that with prompt emergency department POCUS cardiac exam, which in this case revealed acute pericardial effusion and ventricular perforation, earlier identification of cardiac-implant complications may result in decreased morbidity and mortality.
Due to this potentially milder presentation and the difficulty in identifying this condition on imaging, cardiac device lead perforation can be mistaken for a gambit of other cardiac and pulmonary conditions. For this reason, and the estimated 15% of patients with cardiac pacemakers or ICDs who exhibit lead perforation [8], we hypothesize a greater prevalence of mildly symptomatic lead perforation than previously suspected. Particularly in elderly patients, who require a pacemaker or ICD, less severe symptoms such as shortness of breath and fatigue may go unnoticed. As elderly patients also have a higher than average likelihood to exhibit the risk factors associated with cardiac device lead perforation, including low Body Mass Index (BMI), age over 80, and steroid use [3], there is ample cause to give greater clinical consideration to this condition in select patients.
Our patient’s symptoms were appropriately evaluated and correctly attributed to cardiac device lead perforation following bedside POCUS imaging results. Her symptoms in the acute setting are unusual, even amongst the rare reports of acutely symptomatic lead perforation. However, this case suggests that new onset cardiopulmonary symptoms in the first 24 hours after pacemaker or ICD placement require evaluation for lead perforation via bedside POCUS cardiac exam. Even in the absence of the more severe symptoms previously associated with acute lead perforation, this condition should be considered and addressed in a timely manner. Our patient recovered following a lead repositioning procedure without further incident, however, early identification may be key to preventing worse complications in future cases of acute cardiac device lead perforation. Our case serves to inform on this rare presentation of acute cardiac device lead perforation and encourage ED POCUS cardiac exam in patients with nonspecific cardiopulmonary symptoms following cardiac device implantation.
Conclusion
Our patient recovered following a lead repositioning procedure without further incident, however, early identification may be key to preventing worse complications in future cases of acute cardiac device lead perforation. Our case serves to inform on this rare presentation of acute cardiac device lead perforation and encourage ED POCUS cardiac exam in patients with nonspecific cardiopulmonary symptoms following cardiac device implantation
Learning Points
- Atypical presentations of a condition can occur in both the clinical symptomatology and the timing of symptom development
- In patients with an ICD placement who present with chest pain, lead perforation of the myocardium must be included on the differential diagnosis
- POCUS cardiac exam is a useful tool in the ED to diagnose a range of conditions, including cardiac wall perforation
Acknowledgments
This study was not funded.
Competing interests
The authors declare that they have no conflict of interest.
Consent
Patient Consent has been obtained.
Institutional Review Board Statement
N/A, retrospective chart review.
Clinical Trial Registration (Clinical Research Section only)
N/A, retrospective chart review.
Financial Support
None. The authors did not receive grant or outside funding in support of their research or preparation of this manuscript. They did not receive payment or any benefits from commercial entities.
Conflicts of Interest
None. (The authors were not compensated or funded in any way for the preparation of this manuscript. This study has not been submitted elsewhere. We understand and agree that if the manuscript is accepted for publication, copyright in the article, including the right to reproduce the article in all forms and media, shall be assigned to the publisher).
References
- Beyerbach DM (2019) Pacemakers and implantable cardioverter-defibrillators. Medscape, Drugs & Diseases, Cardiology, USA.
- Rajkumar CA, Claridge S, Jackson T, Jonathan B, Johnson J, et al. (2017) Diagnosis and management of iatrogenic cardiac perforation caused by pacemaker and defibrillator leads. Europace 19(6): 1031-1037.
- Mahapatra S, Bybee KA, Bunch TJ, Espinosa RE, Sinak LJ, et al. (2005) Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm 2(9): 907-911.
- Merla R, Reddy NK, Kunapuli S, Rosanio S, Schwarz E, et al. (2007) Late right ventricular perforation and hemothorax after transvenous defibrillator lead implantation. Am J Med Sci 334(3): 209-211.
- Kumar S, Madanieh A, Patel H, Murthy SR, Goyos JM, et al. (2018) Large unilateral pleural effusion with pacemaker-associated post-cardiac injury syndrome. Cureus 10(7): e2946.
- Nichols J, Berger N, Joseph P, Datta D (2015) Subacute right ventricle perforation by pacemaker lead presenting with left hemothorax and shock. Case Rep Cardiol 2015:1-4.
- Vanezis AP, Prasad R, Andrews R (2017) Pacemaker leads and cardiac perforation. JRSM Open 8(3): 205427041668143.
- Hirschl D, Jain V, Spindola-Franco H, Gross J, Haramati L (2007) Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing and Clinical Electrophysiology 30(1): 28-32.
- Xiong M, Zhang Z, Hu K, Dong M, Hu W (2018) Recurrent, late-onset pleural effusions in elderly patients receiving pacemaker therapy. Medicine 97(43): e12915.
- Khalid M, Murtaza G, Ayub MT, Ramu V, Paul T (2018) Right ventricle perforation post pacemaker insertion complicated with cardiac tamponade. Cureus 10(3): e2266.
Citation: Knopp BW, Gerakopoulos K and Parmar J (2022) Acute Pericardial Effusion from Pacemaker Lead Perforation of Right Ventricle Identified on Bedside Emergency Department Ultrasonography. Curr Res Emerg Med 2: 1040