Corpus Journal of Dairy and Veterinary Science
[ ISSN : 2833-0536 ]
Biomechanical Evaluation of a Novel Double-Strand (Looped) Polyamide Monofilament Suture for Canine Flexor Tendon Repair
1Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University,US
2Joint Department of Biomedical Engineering, North Carolina State University and the University of North
Carolina, US
Corresponding Authors
Keywords
Abstract
Objective: Determine the influence of a novel looped polyamide suture on the biomechanical properties and gap formation of repaired canine flexor tendons.
Study Design: Tendons were assigned to 3 groups (n=12/group). Following transection, tendons were repaired with a Kessler pattern using monofilament polypropylene, Kessler pattern using looped polyamide suture and a Kessler pattern using looped polyamide augmented with a continuous Epitendinous Suture (ES) representing groups 1, 2, and 3 respectively. Constructs were tested to simulate clinical failure. Yield, peak and failure loads, loads at -1 and 3mm gap formation and failure modes were analyzed.
Results: Looped polyamide suture is equivalent to monofilament polypropylene using a Kessler core pattern. Looped polyamide suture augmented with a running ES significantly increased yield, peak and failure loads by 3.2x, 3.0x and 2.6x respectively, compared to core suture use alone. Use of an ES required significantly greater force to cause 3mm gap formation while reducing occurrence of gapping in tested constructs. Mode of failure differed among experimental constructs.
Conclusion: Looped polyamide suture is equivalent to monofilament polypropylene in the same pattern. Our results support the addition of ES augmentation, a simple technique modification that demonstrates substantially improved repair strength while reducing the occurrence of gapping between tendon ends. Future in-vivo studies investigating effect of suture placement on tendinous healing, blood supply, and glide function are warranted.
Introduction
Successful tendinous repair in both human and canine patients requires a delicate balance between stability of the repaired construct and controlled post-operative mobility [1]. Components of an ideal tendon repair include ease and consistency of suture placement, minimal extraneous bulk at the repair site, minimal gliding resistance, adequate nutrition and preservation of tendinous blood supply [2]. Factors that maximize tendinous apposition without development of gap formation allows direct contact healing, and the repair be strong enough to resist disruption while allowing adequate strain at the repair site to enhance collagenous remodelling [3]. Two factors shown to increase repair site strength are core suture size utilized [4], and the number of suture strands traversing the repair site [5]. Exploration of newer repair patterns for distal extremity tendon repair techniques have led to modifications in tenorrhaphy suture patterns, increasing the number of core suture strands crossing the repair and suture material choices [6,7]. These factors have led to earlier loading and institution of controlled rehabilitation protocols in affected patients [8]. Use of a single, swaged, looped suture has gained popularity for human tendon repair within recent years [9]. Looped suture use for flexor tendon repair effectively doubles number of suture strands achieved with each suture pass, thereby reducing the number of needle passes necessary to reach the equivalent number of core suture strands crossing the repair site. This design modification simplifies the repair process, reduces the time taken to obtain an equally strong repair and decreased the number of needle punctures to the tendon substance itself [10]. A source of construct weakness is the knot itself [7,11], however few studies have identified the knot as the sole determinant for repair failures when evaluating repair site strength [7,10,11].
Epitendinous repairs consist of a circumferential peripheral suture utilized in combination with a core suture [12]. Suture augmentation prevents fraying and reduces exposure of suture material on the tendon surface thus decreasing gliding friction [12]. Epitendinous Sutures (ES) were recently described within the veterinary literature, shown to be an important structural component, imparting significant strength to the repair while decreasing the occurrence of gap formation [13-16]. Lotz et al. predicted that 64-77% of the load is borne by the ES alone [17]. In a recent study, ES addition to supplement core three-loop-pulley and locking-loop core patterns, significantly increased repair site strength by >250% compared to core suture use alone [15,16]. Achieving anatomic alignment of the epitenon may allow for recruitment and earlier migration of activated fibroblasts to the tendon core, to allow progression of intrinsic healing and earlier return of repair site strength [18]. Several recent studies have led to modifications of core tenorrhaphy patterns, providing higher initial strength to the tendon repair [19]. Recognizing the important biomechanical role played by ES, more attention should be given to improve this aspect of tendinous repairs. To the authors knowledge changes in mechanical properties of the construct by use of a looped tendon suture has not been investigated to date within the veterinary literature. Biomechanical evaluation of novel repair techniques that decrease the risk of repair failures and development of gap formation when physiologic loads are applied to the sutured construct are necessary. Ex-vivo evaluation is required to assess the biomechanical characteristics of ES prior to clinical implementation in canine patients. Creating a tendinous reconstruction that possesses adequate inherent strength to allow for effective, controlled, early rehabilitation, while minimizing associated adverse effects that accompany increasing the number of suture strands is warranted [7].
The objective of this study was to determine the effect of a novel looped polyamide suture with and without a continuous ES in comparison with a core Kessler suture alone on the biomechanical characteristics and gap formation of tested tendon constructs. Our null hypothesis was there would be no difference in the biomechanical properties and loads tolerable at the repair site or development of gap formation between experimental groups in an ex-vivo canine flexor tendon model.
Materials and Methods
Superficial Digital Flexor Tendons (SDFT) were collected from 18 adult mongrel dogs >1 year of age weighing between 28-32 Kg following euthanasia for reasons unrelated to this study. Dogs were obtained following consented donation from a local animal shelter after intravenous euthanasia following infusion of sodium pentobarbital (Euthasol, Virbac AH Inc., Fort Worth, TX, United State). An IACUC protocol was not needed by our institution due to the secondary usage of cadaveric tissues. Dogs were serially examined by a board-certified orthopedic surgeon (DJD) within an hour of euthanasia and excluded if there was evidence of orthopedic disease based on prior examination. Forelimb specimens were dissected yielding a musculotendinous construct that consisted of the distal humeral condyle and associated metaphysis, musculotendinous unit of the SDFT and the tendinous enthesis upon each respective manus. All other surrounding tissues were removed and discarded. Tendon pairs were individually labelled and then wrapped in saline soaked gauze and stored at –20° in an impervious bag (1 Gal, Ziplock, SC Johnson & Son Inc., Racine, WI, United States). Specimens were thawed at room temperature (20 °C) for 12 hours prior to testing. Manual tendinous transection was performed on a hard, durable surface with a #10 scalpel blade at a measured distance of 25 mm distal to the myotendinous junction ensuring a perpendicular sharp transection across the tendinous substance. The distal cut tendon stump was then photographed (iPhoneXR, Apple Inc., CA, United States) at a set distance of 50 mm, held parallel with a calibrated ruler. Tendon CrossSectional Area (CSA) was then calculated by a single study investigator (Y-JC) using a commercially available imaging program (ImageJ, National Institute of Health, Bethesda, MD, United States).
Experimental surgical groups
Using a random sequence generator (Random number generator, https://www. randomizer.org, Research Randomizer, Lancaster, PA, United States), tendons were assigned to 1 of 3 equally sized experimental groups (n=12 constructs/group), while controlling the two forelimbs from each dog being placed within the same group. Group 1 constructs were repaired with a locking Kessler suture as previously described [20], using 2-0 polypropylene (SurgiproTM 2- 0 USP, Covidien Ltd., Dublin, Ireland) on a swaged V-20 26 mm 1/2 circle taper needle. Briefly, sutures were placed equidistant through the center of the tendon with transverse bites taken at a measured distance of 12 mm from the transection site in the distal and proximal stumps respectively. Group 2 constructs were repaired using a novel double-strand (looped) polyamide monofilament suture (Tsuge looped suture, 2-0 USP, Kono Seisakusho Co., Ltd. Chiba, Japan), using a DD 15 mm 3/8 circle taper needle (Figure 1). To start, a longitudinal incision was made at a measured distance of 10 mm from the transected tendon end into the proximal tendon stump using a #10 scalpel blade. At the same level of the incision, on the lateral aspect of the tendon, a small bite was taken then the suture needle was passed through the suture loop, effectively locking the suture in place against the epitenon. The suture was then passed from the level of incision to the tendon end within the core of the tendon substance. In order to prevent the ends of the looped suture from burring into the incision, an intramuscular needle (MonojectTM 20 g, Kendall Healthcare, Mansfield, MA, United States) was utilized to block them. Then a Kessler core suture technique was performed as previously described, in to the distal tendon end at a measured distance of 12 mm from the repair site [20]. Traction was then applied on the suture to close the gap between tendon ends. The looped suture needle was then passed into the proximal tendon end to the level of the longitudinal incision, proximal to the level of the intramuscular needle. One of the two swaged-on suture strands was then cut at the level of the needle.
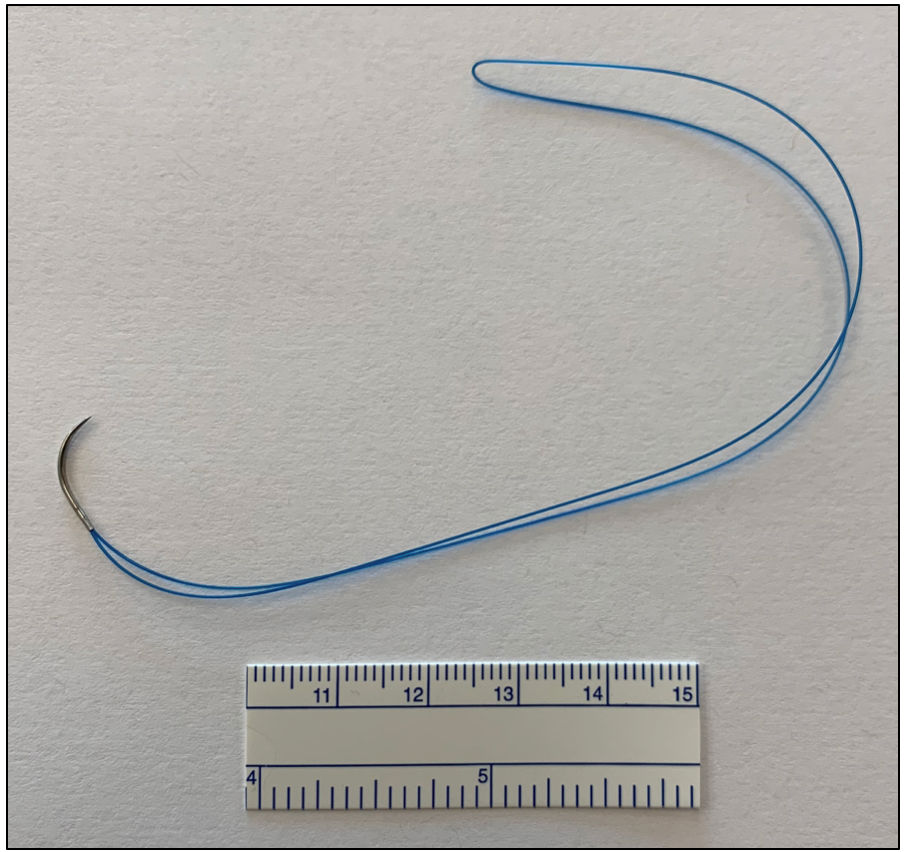
Figure 1: Photograph showing the polyamide looped suture. For scale, a calibrated mm ruler can be seen to the bottom of the image.
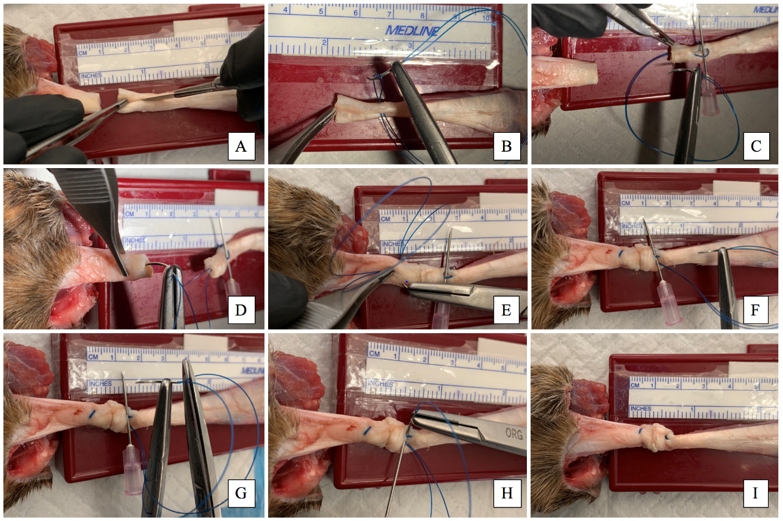
Figure 2: Sequential photographs showing use of the looped suture for a multistrand tendon repair of a canine superficial digital flexor tendon. A: longitudinal incision is made 10 mm from the transection site using a #10 scalpel blade. B: At the same level a small bite was taken then the suture needle was passed through the suture loop, and the suture is locked against the epitenon. C: The suture is then passed from the level of incision to the tendon end. In order to prevent the looped suture from burring into the incision, an intramuscular needle is used to block them. D: A Kessler core suture technique is then used in the distal tendon end at a distance of 12 mm from the transection site. E: Traction is then applied to close the gap between tendon ends. F: The looped suture needle is then passed into the proximal tendon end to the level of the longitudinal incision, proximal to the level of the intramuscular needle. G: One of the two swaged-on suture strands is then cut at the needle. H: The remaining strand attached to the suture needle is passed under the threads following the intramuscular needle. Traction is applied to both suture strands, to close the gap between the tendon ends and the two suture strands were knotted. To hide the knot, both threads were taken proximal to the incision and pulled to bury the knot within the incision and the suture was then cut (not shown in the image). I: A completed 4 strand Kessler flexor tendon repair. As can be seen there is no bunching or plication at the repair site.
Figure 2: Sequential photographs showing use of the looped suture for a multistrand tendon repair of a canine superficial digital flexor tendon. A: longitudinal incision is made 10 mm from the transection site using a #10 scalpel blade. B: At the same level a small bite was taken then the suture needle was passed through the suture loop, and the suture is locked against the epitenon. C: The suture is then passed from the level of incision to the tendon end. In order to prevent the looped suture from burring into the incision, an intramuscular needle is used to block them. D: A Kessler core suture technique is then used in the distal tendon end at a distance of 12 mm from the transection site. E: Traction is then applied to close the gap between tendon ends. F: The looped suture needle is then passed into the proximal tendon end to the level of the longitudinal incision, proximal to the level of the intramuscular needle. G: One of the two swaged-on suture strands is then cut at the needle. H: The remaining strand attached to the suture needle is passed under the threads following the intramuscular needle. Traction is applied to both suture strands, to close the gap between the tendon ends and the two suture strands were knotted. To hide the knot, both threads were taken proximal to the incision and pulled to bury the knot within the incision and the suture was then cut (not shown in the image). I: A completed 4 strand Kessler flexor tendon repair. As can be seen there is no bunching or plication at the repair site. as for group 2 constructs. After completion of the core pattern, a running continuous ES was placed as previously described [15,16]. The epitendinous suture utilized 3-0 polypropylene (SurgiproTM 3- 0 USP, Covidien Ltd., Dublin, Ireland) on a swaged V-20 26 mm 1/2 circle taper needle placed 10 mm from either end of the transection site. In all groups a single square knot followed by 3 throws was used for Kessler and ES patterns after drawing tendinous ends together into close apposition without occurrence of bunching at the repair site. Although determined subjectively, equal tension was placed prior to tying the knot in all experimental constructs. Suture strands were cut to a length of 3 mm. A single board-certified surgeon (DJD) performed all surgical repairs and specimen testing.
Tensile testing
Experimental testing was performed using a materials testing machine (Instron 5944, Instron, Norwood, MA, United States) at 21 °C within a thermostatically controlled environment. Following pattern completion, the distal humeri was secured to the custom testing jig, with a 4.5 mm stainless steel bolt placed transversely through the supratrochlear foramen of the humerus. Specimens were then affixed to a 500 N load cell mounted on the cross-head of the testing machine. A digital high definition camera (Brio 4k Webcam, Logitech, Silicon Valley, CA, United States) was positioned at a measured distance of 25 cm from the construct to record each individual test. A graduated mm ruler was axially aligned within the viewing window of the camera adjacent to constructs during data recording (Figure 3). The manus was then mounted and rigidly affixed using a bone clamp (SKU 1652-1, Sawbones, Vashon Island, WA, United States). Forelimb specimens were vertically aligned during testing to mimic the position of the canine forelimb splinted in the immediate post-operative period. Pre-loading of the constructs then progressed to 2 N with all measurements zeroed and then the machine calibrated to ensure a consistent resting length among specimens. Constructs were then distracted until catastrophic failure at a rate of 20 mm/minute. Load (Newtons, N) and displacement (mm) data was collected and viewed within a software program (Bluehill 3, Instron Inc., Norwood, MA, United States) at a frequency of 100 Hz. Load at yield was defined as the greatest force achieved prior to any initial decrease in the load-displacement curve. Peak load was defined as the maximum force measured during each test. Failure load was defined as the point at which the suture broke or pulled-through the tendinous tissue, or when there was a sharp decrease in the load-displacement curve. Failure method was documented visually at the time of testing and confirmed by retroactive review of frame data by a single study investigator (Y-JC.
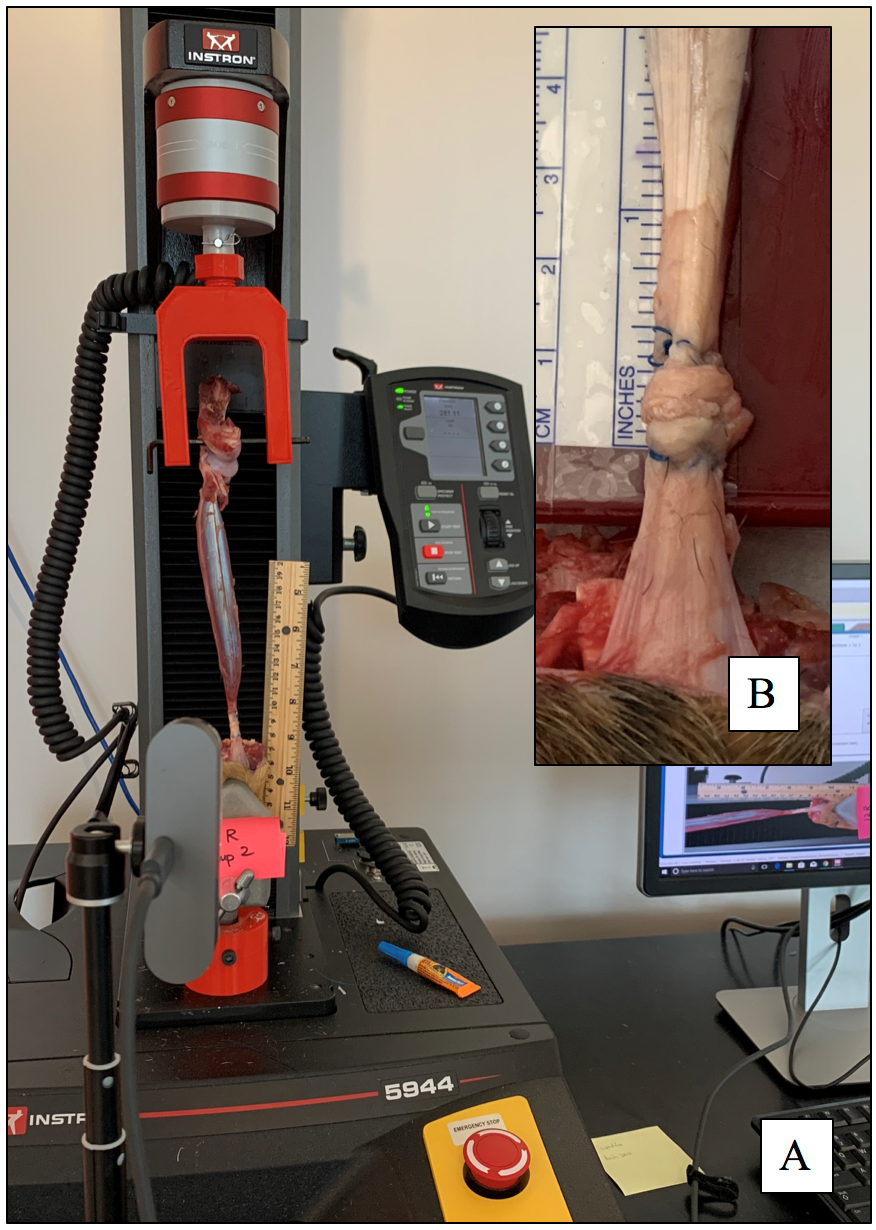
Figure 3: A: Mechanical tensile testing apparatus with a repaired flexor tendon b construct loaded within the custom testing apparatus. Construct shows a core Kessler repair with a looped suture technique. B: Insert shows magnified photographic image of the repair site with a calibrated ruler seen adjacent to the image.
Graphical load-displacement curves were generated to allow the force (N) at yield, peak and failure loads for each construct test to be calculated. A custom program (Matlab R2018b, Mathworks, Natick MA, United States) was utilized to assist with precise selection of these data points. High-speed recordings were reviewed following testing to evaluate for the development of the smallest distance between tendon ends to measure for -1- and 3 mm gaps respectively. The ruler placed within the viewing window was used to calibrate a digital caliper for each construct using an imaging software program (ImageJ, National Institute of Health, Bethesda, MD, Unites States). Frame data was assessed for each construct to determine the time points and respective loads at which -1 and 3 mm gaps developed between tendon ends at the transection site.
Statistical analysis
A priori power analysis was performed following completion of a pilot study using 3 dogs. Based on pilot data and failure loads of a prior study [21], a prospective power calculation determined that at least 12 tendons per group would provide at least 90% power to detect a mean difference between groups of 30 N ± 5 N at a 5% alpha error rate in independent measures. Pilot data was not included within the final analysis. Data was assessed for parametric distribution using the Shapiro-Wilk test for normality. Continuous variables were normally distributed and described using mean ± standard deviation. One-way analysis of variance was used to compare the continuous variables between groups. Pairwise comparison was performed by using the Tukey-Kramer test. Proportional distributions in failure mode were compared between experimental groups using the Pearson chi-square test of association. Statistical analyses were performed using commercially available software (Statistical software, JMP Pro 14, SAS Institute Inc, Cary, NC, United States). A p-value of <0.05 was considered statistically significant.
Results
Tendon data
All repaired constructs were sutured and tested without observed error, with all tendons included within the final statistical model. Left and right limbs were equally distributed among experimental groups. Mean tendon CSA was 0.26 ± 0.04 cm2 and did not differ between experimental groups (p = 0.648).
Load data
Yield, peak, and failure load data is summarized in Table 1. Yield load was significantly different between experimental groups (p < .0001). When comparing group 1 (44.02 ± 9.55 N), group 2 (51.38 ± 32.17 N) and group 3 (139.78 ± 35.32 N) constructs, yield load differed between groups 1 and 3 (p < .0001), 2 and 3 (p < .0001) but not groups 1 and 2 (p = 0.799), respectively. Peak load also differed among experimental groups (p < .0001). When comparing failure load, group 1 (53.89 ± 6.85 N), group 2 (65.33 ± 21.74 N) and group 3 (141.07 ± 21.48 N) constructs differed between groups 1 and 3 (p < .0001), 2 and 3 (p < .0001) but not group 1 and 2 (p = 0.282), respectively (Figure 4).
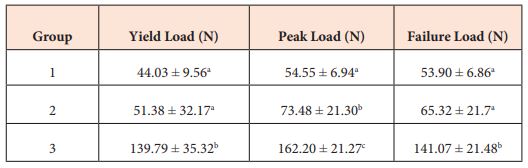
Table 1: Yield, peak, and failure loads in Newtons (N) for canine superficial digital flexor tendon repairs showing different experimental groups. Group 1 – Kessler alone; Group 2 – Novel polyamide looped suture using a modified Kessler pattern; Group 3 – Looped Kessler with a running continuous epitendinous suture. Data is reported as Mean ± SD. There was a significant difference in yield, peak and failure loads (P < .0001). Yield, peak and failure loads differed significantly between groups 1 and 3 (p < .0001), 2 and 3 (p < .0001). Groups 1 and 2 differed significantly regarding loads at peak force (p = 0.038) but not in yield (p = 0.799) and failure loads (p = 0.282). Different superscript letters denote significant differences between groups.
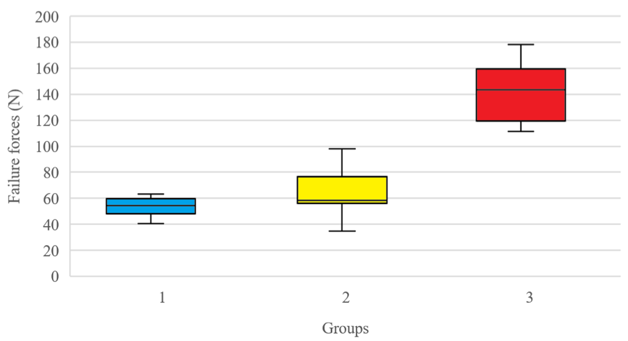
Figure 4: Box and whisker plot depicting failure force of tenorrhaphies repaired with Group 1 – Kessler pattern using a monofilament polypropylene; Group 2 – Kessler pattern using a looped polyamide suture; Group 3 – Kessler pattern using a looped polyamide suture augmented with a running continuous epitendinous suture. Regarding failure load, group 1 (53.89 ± 6.85 N), group 2 (65.33 ± 21.74 N) and group 3 (141.07 ± 21.48 N) constructs differed between groups 1 and 3 (p < .0001), 2 and 3 (p < .0001) but not group 1 and 2 (p = 0.282), respectively. Boxes represent interquartile range, the horizontal line in each box represents the median, whiskers extend to the highest and lowest values, and circles represent outliers.
Gap formation between tendon ends
Load required to create a 1 mm gap between tendon ends was significantly different between experimental groups (p < .0001). When comparing load to cause a 1 mm gap, there was a significant difference between groups 1 and 3 (p < .0001), 2 and 3 (p < .0001), but not groups and 1 and 2 respectively (p = 0.281). Similarly, load application to create a 3 mm gap was significantly different between groups (p < .0001). When comparing load to cause a 3 mm gap formation, there was a significant difference between groups 1 and 3 (p < .0001), 2 and 3 (p < .0001), but not groups and 1 and 2 respectively (p = 0.229). Gap formation data is shown in Table 2.
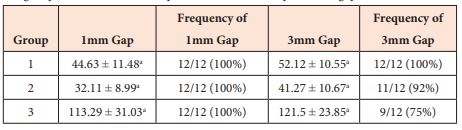
Table 2: load required in Newtons (N) to cause occurrence of -1 and 3 mm gaps and frequency (%) of gap formation between tendon ends. Superscripts letters denote significant differences between experimental groups (p < 0.001). Load to create a 1 and 3 mm gap was significantly greater for group 3 (p < 0.0001). Occurrence of gap formation differed significantly between groups 1 and 3 (p < 0.0001), 2 and 3 (p < 0.0001) but not groups 1 and 2 for both -1 (p = 0.282) and 3 mm (p = 0.229) gap formation forces.
Failure mode
Mechanism of construct failure among all tested specimens included suture breakage or suture pull-through of the core suture. Failure mechanisms differed significantly between experimental groups (p < .0004) with suture breakage being the most common mode of failure in 9/12 (75%) of group 1 constructs. For group 2 the predominant mode of failure was suture pull-through in 11/12 (92%) constructs. In group 3 specimens constructs failed predominantly by core suture breakage in 7/12 (58%) with the running continuous ES failing by suture breakage in 12/12 (100%) of repairs.
Discussion
The findings of our study indicate the use of looped polyamide suture in a Kessler pattern augmented with a running continuous ES significantly increases yield, peak and failure loads by 3.2x, 3.0x and 2.6x respectively, compared to use of a core Kessler pattern using polypropylene alone. Use of a looped suture with addition of an ES required significantly greater force to cause 3 mm gap formation between tendons ends while reducing occurrence of 3 mm gapping in tested constructs. Results of this study support the use of a looped polyamide suture for canine flexor tendon repair with the addition of an ES, a technique modification that significantly increases the biomechanical strength and improves gapping characteristics of tested repairs. Conceptual change to human flexor tendon repairs techniques have shown multistrand repairs using looped or multiple sutures to be employed [22,23] Prior studies have led to technique modifications and patterns using looped suture [23-26] Multistrand suture techniques have been advocated for as they can reduce surgical repair times, reduce pattern complexity, improve reproducibility while reducing necessity for tendon manipulation and collagen fiber disruption within the tendon core [22]. Several disadvantages regarding the use of looped sutures should also be recognized. Suture knotting may result in excessive bulk or multiple knots may be required. Ensuring equal tension of each respective suture arm at the time of suture knotting can be challenging, even under experimental ex-vivo conditions. Similar concerns have been raised for anterior cruciate ligamentous reconstruction using a multi-strand repair [27]. A study by Brockardt et al. [24] reported that a 4 strand Kessler repair using a looped suture was inferior to use of 2 separately placed Kessler patterns using a single suture strand. It should be noted however, that their study did not evaluate patterns augmented with an ES. Within our study, use of a novel polyamide looped suture was equivalent to a polypropylene suture of similar size. With the addition of an epitendinous suture yield, peak and failure loads tolerated at the repair site increased by 3.2x, 3.0x and 2.6x respectively, compared to use of a core Kessler pattern (group 1) alone. Loads experienced by individual musculotendinous units by dogs during different phase of the gait cycle is currently unknown. Ground reaction forces (G) placed upon the thoracic limb at the walk however can be assumed to be equal to 30% of BW [3,28]. Thus, for an equivalent 30 kg dog, G approximates nearly 90 N. In our study the use of looped suture augmented with an ES exceeded this value, suggesting this novel suture may offer an viable alternative to conventional tenorrhaphy techniques currently utilized by small animal surgeons.
Gap formation is an undesirable trait following tendinous repair. Gap formation leads to production of a disorganized and mechanically inferior scar between tendon ends [3]. Once a gap is >3mm, force experienced by the repair dramatically increases, quickly reaching its ultimate failure strength [3]. Elongation has also been associated with adhesion formation, impaired healing, predisposition to repair failure and poor functional outcomes following acute surgical intervention [3]. In our study, occurrence of gap formation between tendon ends was similar between repairs using a single strand monofilament and the polyamide looped suture. Incidence of gap formation was significantly reduced when an epitendinous suture was added to augment the core repair and agrees with the findings of prior studies [18,29-31]. Although subjectively assessed, when suturing tendinous constructs we found that the Kessler core pattern, using both single suture strands and the looped suture, there was minimal bunching at the repair site and uniform apposition of the tendon ends at the repair site. We elected to choose a running continuous ES based on the results of previous studies [12,16]. Significant improvements regarding tensile strength of sutured constructs in conjunction with being more resistant to gap formation, may translate to superior apposition between tendon ends and collagen fibril alignment in-vivo leading to earlier accrual of repair site strength. A prospective clinical trial is necessary to further elucidate these hypotheses. Predisposition to repair failure and ultimate repair site strength depend upon the suture utilized and its holding capacity and interaction within the tendon substance. If the suture represents the weaker component, repairs will fail predominantly by suture breakage. If suture holding capacity within the tendon is inferior, repairs will fail by suture pull-through. In our study mode of failure differed among experimental groups. Suture breakage was most common among core Kessler sutures using monofilament polypropylene which differed from repairs using a core looped suture which failed by mechanism of suture failure. Based on these observations this indicates that the weakest element among repair using a looped suture is the tendon substance and not the suture material. Postulated ways to increase construct strength may be to position the cross-stitch of the Kessler suture further from the transection site [32]. Tendon strength has been shown to be proportional to the number of longitudinal suture strands crossing the transection site [33-36]. with tensile strength also affected by the size of the locking loops of the Kessler pattern, with grasping force is in direct relation to the loop diameter [36]. Postulated ways to further increase construct strength maybe to place 2 Kessler core sutures as previously described [21], however instead using the looped suture, so now 8 strands would traverse the repair site rather than 4 as used in this model. Use of an ES agrees with previous studies showing the positive effect of epitendinous placement on construct strength and resistance to deformation [13-16]. Another method to resist suture pullthrough may be utilization of core barbed sutures in a similar pattern to maximize interaction of barbs with collagen fibrils within the tendon. Future work is necessary to elucidate the most appropriate size of the epitendinous suture utilized in dogs to maximize repair site strength while not adversely affecting tendon nutrition and blood supply.
This ex-vivo biomechanical study has some inherent limitations unique to its experimental design. We harvested canine tendons that were assessed to be healthy and were sharply transected prior to testing. We appreciate this differs from cases in-vivo where chronic degenerative changes result in fraying of the tendon ends with diminished suture holding capacity. Due to testing of cadaveric tissues, important biological factors such as local tissue ischemia, edema, and adhesion formation that can occur during the normal healing process were not assessed. Relevant factors invivo were not assessed including the effect of tissue or suture reactivity, infection, the acute inflammatory response, effect of utilized patterns on tendinous blood supply, as well assessment of glide-function. In our study, experimental sutures utilized for this study were produced by different manufacturers, formed from different materials, and used different suture needles. These variables were however deliberate to appropriately represent what is currently performed clinically and products currently available for tendinous repair within veterinary medicine. For the purpose of this biomechanical analysis, we used axial distraction to represent acute failure in the immediate postoperative period without evaluation of cyclical loading, which more accurately represents clinical conditions [37]. Lastly, we did not compare use of a single Kessler suture using polypropylene with the addition of an ES. Prior studies [15,16], have demonstrated the beneficial effects of ES application in conjunction with commonly utilized patterns using similar loading protocols to allow direct comparisons to be drawn between studies.
Conclusion
This study shows use of a novel looped polyamide suture to be equivalent to use of monofilament polypropylene in the same pattern. Addition of a running continuous ES to a primary Kessler repair significantly increased yield, peak and failure loads tolerated at the repair site while reducing the occurrence of gapping between tendon ends. Success of the instituted repair technique relies upon many different factors such as the patient and owner compliance to post-operative instructions and confinement, institution of controlled tendon loading and rehabilitation. Future studies investigating the effect of looped sutures along with epitendinous augmentation are necessary to evaluate for progression of tendon healing, blood supply, and glide function are warranted to determine their effectiveness and practicality in a clinical setting following flexor tendon injury.
Acknowledgement
All surgical sutures used for the purpose of this study was kindly donated by Medtronic, Inc., Mansfield, MA and Crownjun, Kono Co, Japan. These medical manufacturers had no role in the study design, data collection and analysis, decision to publish the experimental data, or preparation of the resultant manuscript. The authors declare no conflict of interest related to this study, nor was any financial support received.
References
- Frank CB, Shrive NG, Lo IKY, Hart DA (2007) Form and function of tendons and ligaments. In: O'Keefe RJ, Buckwalter JA, Einhorn TA (Eds.), Orthopaedic basic science : Foundations of clinical practice. (3rd edn), Rosemont, IL : American Academy of Orthopaedic Surgeons, US, pp.191-222.
- Boyer MI, Goldfarb CA, Gelberman RH (2005) Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther Off 18(2): 80-85.
- Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ (1999) The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am 81(7): 975-982.
- Peltz TS, Haddad R, Scougall PJ, Nicklin S, Gianoutsos MP, et al. (2011) Influence of locking stitch size in a four-strand cross-locked cruciate flexor tendon repair. J Hand Surg 36(3): 450-455.
- Rees L, Matthews A, Masouros SD, Bull AMJ, Haywood R (2009) Comparison of 1- and 2-knot, 4-strand, double-modified kessler tendon repairs in a porcine model. J Hand Surg 34(4):705-709.
- Wu YF, Tang JB (2014) Recent developments in flexor tendon repair techniques and factors influencing strength of the tendon repair. J Hand Surg Eur 39(1): 6-19.
- Dy CJ, Hernandez-Soria A, Ma Y, Roberts TR, Daluiski A (2012) Complications after flexor tendon repair: A systematic review and meta-analysis. J Hand Surg 37(3): 543-551.
- Kim HM, Nelson G, Thomopoulos S, Silva MJ, Das R, et al. (2010) Technical and biological modifications for enhanced flexor tendon repair. J Hand Surg 35(6): 1031-1037.
- Gil JA, Skjong C, Katarincic JA, Got C (2016) Flexor tendon repair with looped suture: 1 versus 2 knots. J Hand Surg 41(3): 422-426.
- Netscher DT, Badal JJ, Yang J, Kaufman Y, Alexander J, et al. (2015) Biomechanical evaluation of double-strand (looped) and single-strand polyamide multifilament suture: influence of knot and suture size. Hand N Y N 10(3): 417-424.
- McClellan WT, Schessler MJ, Ruch DS, Levin LS, Goldner RD (2011) A knotless flexor tendon repair technique using a bidirectional barbed suture: an ex vivo comparison of three methods. Plast Reconstr Surg 128(4): 322e-327e.
- Wieskötter B, Herbort M, Langer M, Raschke MJ, Wähnert D (2018) The impact of different peripheral suture techniques on the biomechanical stability in flexor tendon repair. Arch Orthop Trauma Surg 138(1): 139-145.
- Duffy DJ, Chang YJ, Gaffney LS, Fisher MB, Moore GE (2019) Effect of bite depth of an epitendinous suture on the biomechanical strength of repaired canine flexor tendons. Am J Vet Res 80(11): 1043-1049.
- Duffy DJ, Cocca CJ, Kersh ME, Kim W, Moore GE (2019) Effect of bite distance of an epitendinous suture from the repair site on the tensile strength of canine tendon constructs. Am J Vet Res 80(11): 1034-1042.
- Putterman AB, Duffy DJ, Kersh ME, Rahman H, Moore GE (2019) Effect of a continuous epitendinous suture as adjunct to three-loop pulley and locking-loop patterns for flexor tendon repair in a canine model. Vet Surg 48(7): 1229-1236.
- Cocca CJ, Duffy DJ, Kersh ME, Kim W, Groenewold A, et al. (2019) Biomechanical comparison of three epitendinous suture patterns as adjuncts to a core locking loop suture for repair of canine flexor tendon injuries. Vet Surg 48(7): 1245-1252.
- Lotz JC, Hariharan JS, Diao E (1998) Analytic model to predict the strength of tendon repairs. J Orthop Res Off Publ Orthop Res Soc 16(4): 399-405.
- Fufa DT, Osei DA, Calfee RP, Silva MJ, Thomopoulos S, et al. (2012) The effect of core and epitendinous suture modifications on repair of intrasynovial flexor tendons in an in vivo canine model. J Hand Surg 37(12): 2526-2531.
- Takeuchi N, Mitsuyasu H, Hotokezaka S, Miura H, Higaki H, et al. (2010) Strength enhancement of the interlocking mechanism in cross-stitch peripheral sutures for flexor tendon repair: biomechanical comparisons by cyclic loading. J Hand Surg Eur 35(1): 46-50.
- Wade PJ, Muir IF, Hutcheon LL (1986) Primary flexor tendon repair: the mechanical limitations of the modified Kessler technique. J Hand Surg Edinb Scotl 11(1): 71-76.
- Moores AP, Owen MR, Tarlton JF (2004) The three-loop pulley suture versus two locking-loop sutures for the repair of canine achilles tendons. Vet Surg 33(2): 131-137.
- Strickland JW (2000) Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg 25(2): 214-235.
- Tang JB, Shi D, Gu YQ, Chen JC, Zhou B (1994) Double and multiple looped suture tendon repair. J Hand Surg Edinb Scotl 19(6): 699-703.
- Brockardt CJ, Sullivan LG, Watkins BE, Wongworawat MD (2009) Evaluation of simple and looped suture and new material for flexor tendon repair. J Hand Surg Eur 34(3): 329-332.
- Brockardt CJ, Sullivan LG, Watkins BE, Wongworawat MD (2009) Evaluation of simple and looped suture and new material for flexor tendon repair. J Hand Surg Eur 34(3): 329-332.
- Gill RS, Lim BH, Shatford RA, Toth E, Voor MJ, et al. (1999) A comparative analysis of the six-strand double-loop flexor tendon repair and three other techniques: A human cadaveric study. J Hand Surg 24(6): 1315-1322.
- Hamner DL, Brown CH, Steiner ME, Hecker AT, Hayes WC (1999) Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am 81(4): 549-557.
- Lascelles BDX, Roe SC, Smith E, Reynolds L, Markham J, et al. (2006) Evaluation of a pressure walkway system for measurement of vertical limb forces in clinically normal dogs. Am J Vet Res 67(2): 277-282.
- Chauhan A, Schimoler P, Miller MC, Kharlamov A, Merrell GA, et al. (2018) Comparing Biomechanical Properties, Repair Times, and Value of Common Core Flexor Tendon Repairs. Hand N Y N 13(3): 313-318.
- Lin GT, An KN, Amadio PC, Cooney WP (1988) Biomechanical studies of running suture for flexor tendon repair in dogs. J Hand Surg 13(4): 553-558.
- Klifto CS, Capo JT, Sapienza A, Yang SS, Paksima N (2018) Flexor tendon injuries. J Am Acad Orthop Surg 26(2): e26-e35.
- Gil-Santos L, Monleón-Pradas M, Gomar-Sancho F, Más-Estellés J (2018) Positioning of the cross-stitch on the modified Kessler core tendon suture. J Mech Behav Biomed Mater 80: 27-32.
- Barrie KA, Wolfe SW, Shean C, Shenbagamurthi D, Slade JF, et al. (2000) A biomechanical comparison of multistrand flexor tendon repairs using an in situ testing model. J Hand Surg 25(3): 499-506.
- Cao Y, Zhu B, Xie RG, Tang JB (2006) Influence of core suture purchase length on strength of four-strand tendon repairs. J Hand Surg 31(1): 107-112.
- Winters SC, Gelberman RH, Woo SL, Chan SS, Grewal R, et al. (1998) The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: A biomechanical study in dogs. J Hand Surg 23(1): 97-104.
- Xie RG, Xue HG, Gu JH, Tan J, Tang JB (2005) Effects of locking area on strength of 2- and 4-strand locking tendon repairs. J Hand Surg 30(3): 455-460.
- Pruitt DL, Manske PR, Fink B (1991) Cyclic stress analysis of flexor tendon repair. J Hand Surg 16(4): 701-707.
Citation: Duffy DJ, Chang YJ, Chiu KW and Fisher MB (2022) Biomechanical Evaluation of a Novel Double-Strand (Looped) Polyamide Monofilament Suture for Canine Flexor Tendon Repair. Corpus J Vet Dairy Sci 3: 1044