Current Research in Emergency Medicine
[ ISSN : 2832-5699 ]
Myocardial Involvement in Systemic Lupus Erythematosus: Not only A Problem of Epicardial Coronary Artery
1Cardiology Department Guglielmo Da Saliceto Hospital; Piacenza, Italy
2Department of Clinical and Experimental Medicine, University of Parma, Parma, Italy
Corresponding Authors
Keywords
Abstract
42-year-old woman, active smoker, with an history of arterial hypertension and SLE, treated with Prednisone and Hydroxychloroquine, was admitted to the Emergency Department (ED) complaining of severe chest pain. Electrocardiogram revealed normofrequent sinus rhythm with negative/diphasic T waves in Infero-lateral leads. Bedside Transthoracic Echocardiogram (TTE) showed mild impairment of ventricular systolic function (EF 50%) in presence of hypokinesia of the anterior wall in mid- basal segment. Cardiac biomarkers were elevated and a diagnosis of acute coronary syndrome without persistent ST Elevation Myocardial Infarction (ASC N-STEMI) was performed. The patient underwent for coronary angiography that revealed coronary arteries free from significant atherosclerotic lesions. Moreover, Cardiac Magnetic Resonance (CMR) showed signs of hypertrophic cardiomyopathy along with T2 hyperintensity and mesocardial Late Gadolinium Enhancement (LGE) in the basal segment of postero- lateral wall consistent with acute myocarditis.
Learning points:
cardiac involvement in Systemic Lupus Erythematosus (SLE) patient’ is frequent and it doesn’t depend only on chronic inflammation and autoimmune disregulation.
Introduction
Systemic Lupus Erythematosus (SLE) is an uncommon and idiopathic autoimmune disease, with an estimated prevalence of 1/2000 and a male : female ratio of 1:10. Cardiac involvement could be in form of myocarditis, pericarditis, Libman Sachs endocarditis, vasculopathy secondary to antiphospholipid antibody syndrome, vasculitis or Coronary Artery Disease (CAD) [1]. The increased prevalence of coronary heart disease and cardiovascular mortality in patients with SLE cannot be explained only by the traditional risk factors for atherosclerosis [2]. The premature atherosclerosis is closely related to the chronic inflammatory process and to autoimmune dysregulation. Therefore, pro-inflammatory cytokines are responsible for pro-atherogenic alterations that include endothelial dysfunction, insulin resistance and a prothrombotic effect.
Case Presentation
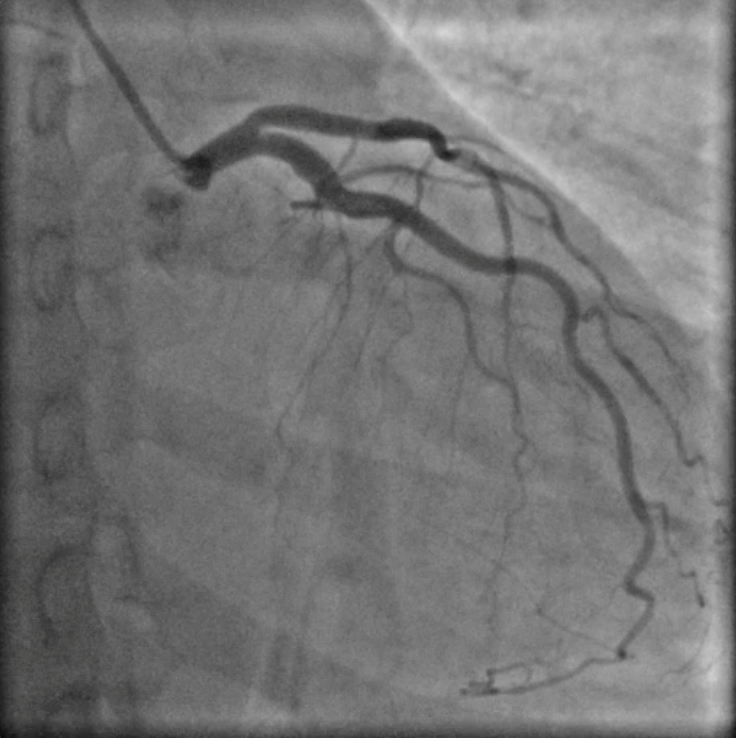
Figure 1: coronary angiography with coronary arteries free from significant atherosclerotic lesions
A 42-year-old woman, active smoker, with an history of arterial hypertension well controlled with medical therapy (mean arterial pressure of 130/80 mmHg with valsartan 160 mg/die) and SLE diagnosed in 2017, associated to leuco-neuthropenia (white blood cell 2260 x 103 /uL for a normal 4000-10000 x 103 /uL , neuthrofilis 990/uL for a normal 1.60-7.70 x 103/uL), with no extra-cardiac lupus symptoms and treated with Prednisone and Hydroxychloroquine (HCQ) with negativation of inflammatory parameters (C-reactive protein 1.62 mg/L for a normal 0.001-5 mg/L, erythrocyte sedimentation rate 28 mm for a norma 2-30 mm). She was admitted to the Emergency Departement (ED) for severe chest pain. Clinical observation showed blood pressure 145/85 mmHg, regular heart sounds, no heart murmurs, arterial oxygen saturation of 97%. Electrocardiogram revealed normofrequent sinus rhythm with negative /diphasic T waves in infero-lateral leads. Bedside Transtoracic Echocardiography (TTE) showed mild impairment of ventricular systolic ejection function (EF 50%) with hypokinesia of the anterior wall in mid-basal segment. Cardiac biomarkers were elevated (high sensitivity troponin I 756.1 ng/L at the first detection and 5079.3 ng/L at the second control, for a normal 10,5 ng/L). A diagnosis of acute coronary syndrome with non-ST segment elevation myocardial infarction (ASC N-STEMI) was performed. The patient underwent for coronary angiography that revealed coronary arteries free from significant atherosclerotic lesions as shown in Figure 1 Moreover Cardiac Magnetic Resonance (CMR) showed signs of hypertrophic cardiomyopathy with mildly impairment of left ventricular global function (EF 45%). On T2 weighed sequences there was mesocardial hyperintensity in basal segment of postero-lateral wall. On T1 weighed sequences with paramagnetic contrast there was mesocardial LGE in same position consistent with acute myocarditis, as shown in Figure 2. At last laboratory screening tests for antiphospholipid antibody syndrome resulted within limits. These findings support the diagnosis of acute myocarditis in the setting of HCQ-induced cardiomyopathy.
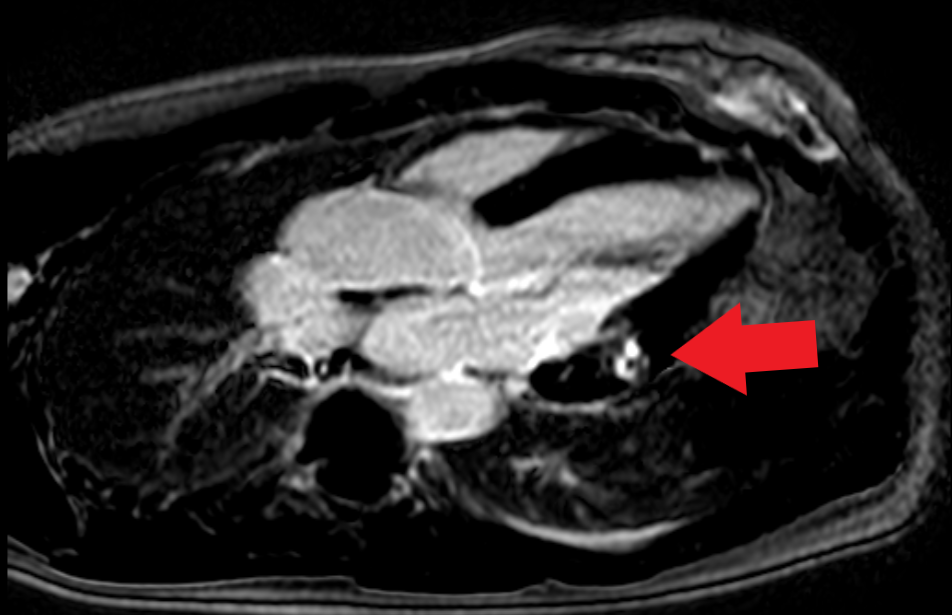
Figure 2: CMR with mesocardial LGE.
Discussion
Although uncommon, myocarditis is a serious manifestation of SLE, with a clinical prevalence of about 9%, although post-mortem studies suggest a higher prevalence of subclinical myocarditis (about 57%) [3]. Typical clinical findings are dyspnea, nonexertional chest pain, peripheral edema, fever, orthopnea, diaphoresis, paroxysmal nocturnal dyspnea, nausea, vomiting, or palpitations. Endomyocardial biopsy could confirm the diagnosis of myocarditis but sensitivity and specificity are very low due to the focal involvement of myocardium. Histologically findings are patches of myocardial fibrosis, interstitial mononuclear cell infiltrates and myocyte necrosis with immune complex deposition, even in areas not involved from inflammatory process. The diagnosis is often achieved clinically and with the use of diagnostic imaging. CMR can also detect the presence of necrosis or scar in myocarditis by late gadolinium enhancement.
18F-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography (18F-FDG PET-CT) has a novel and more sensitive imaging modality for active inflammation. To date 18F-FDG PET-CT has few data in the evaluation of myocardial inflammation in SLE patient’s and observational studies are needed [4]. The absence of disease of epicardial coronary arteries showed the importance of coronary artery microcirculation. To date many connective diseases are associated to endothelial dysfunction. Notably the endothelium is responsible for the continuous adjustment of vascular tone through the balance between the vasodilators (e.g. prostacyclin/PGI2 nitric oxide/NO, endothelium-derived hyperpolarizing factor and carbon monoxide/CO) and the vasoconstrictors (e.g. endothelin-1, thromboxane A2 and endoperoxidase), control of blood pressure, regulation of leucocyte traffic from blood to tissues and the maintenance of antithrombotic and anticoagulant balance in flowing blood [5]. Pro-inflammatory mediators such as Tumors Necrosis Factor (TNF)-α, are strongly linked to endothelial activation and dysfunction [6]. Persistent endothelial activation leads to endothelial dysfunction, which is characterized by loss of vascular integrity, change in phenotype from antithrombotic to thrombotic, reduced production of nitric oxide and increased expression of adhesion molecules and chemokines, resulting in impaired vasorelaxation and recruitment of blood-borne mononuclear cells such as monocytes and T lymphocytes [7]. Furthermore, endothelial dysfunction is associated with an increased risk of developing atherosclerosis and cardiovascular disease, which reduce life expectancy of these patients by 10–15 years [8]. Experimentally SLE women patient’s symptomatic for chest pain underwent for adenosine stress CMR that showed abnormal stress myocardial perfusion. This is probably due to myocardial ischemia caused by microvascular coronary dysfunction [9]. During five years of follow-up the majority of SLE patient’s who have persistent chest pain showed similar or worse myocardial perfusion at CMR, consistent with Coronary Microvascular Dysfunction without obstructive coronary artery disease [10].
Furthermore, severe cardiac toxicity due to prolonged use of HCQ included cardiomyopathy. The mechanism of cardiotoxicity probably due to the alkalization of cardiomyocyte lysosomes and thus inhibition of the activity of alpha-galactosidase A, which leads to intracellular accumulation of polysaccharides [11]. Clinically HCQinduced cardiomyopathy manifested as diffusely thickened restrictive or dilated cardiomyopathy or with conduction system abnormalities including atrioventricular block and bundle branch block [12]. CMR is a useful instrument to evaluate biventricular function, the extent of myocardial hypertrophy and fibrosis. Only endomyocardial biopsy could confirm the diagnosis of HCQ-induced cardiomyopathy underlying typical pathological changes including lamellar and curvilinear bodies [13].
Finally, steroid treatment itself promote a condition of hyperinsulinemia and insulin resistance, that correlates with the severity of the inflammatory process, and it could contribute to the development of ischemic heart disease.
Conclusion
Among SLE patient’s chest pain is often challenging and We have to keep in mind that the disease may involve not only epicardial coronary arteries. Myocarditis and HCQinduced cardiomyopathy are uncommon but severe complication. Chronic inflammation and autoimmune dysregulation could lead to premature atherosclerosis and micro vascular disfunction. CMR along with stress protocol is a useful diagnostic tool.
Disclaimer:
None
Consent for publication:
All the patients involved in the current manuscript gave their consent for publication
Acknowledgement:
None
Conflict of interest:
None declared.
Funding:
None
References
- Mattu A, Petrini J, Swencki S, Chaudhari C, Brady WJ (2005) Premature atherosclerosis and acute coronary syndrome in systemic lupus erythematosus. Am J Emerg Med 23(5): 696-703.
- Roman M, Shanker BA, Davis A, Lockshin MD, Sammaritano L (2003) Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med 349(25): 2399-2406.
- Bulkley BH, Roberts WC (1975) The heart in systemic lupus erythematosus and the changes induced in it by corticosteroid therapy. A study of 36 necropsy patients. Am J Med 58(2): 243-264.
- Winkler AP, Bokhari S, Perez-Recio T, Zartoshti A, Askanase A et al. (2018) Myocarditis in systemic lupus erythematosus diagnosed by F-fluorodeoxyglucose positron emission tomography. Lupus Sci Med 5(1): e000265.
- Pearson JD (2000) Normal endothelial cell function. Lupus 9(3): 183-188.
- Pober JS (2002) Endothelial activation: intracellular signaling pathways. Arthritis Res 4(suppl 3): S109-S116.
- Ross R (1999) Atherosclerosis – an inflammatory disease. N Engl J Med 340(2): 115-126.
- Wang L, Feng G (2004) Rheumatoid arthritis increases the risk of coronary heart disease via vascular endothelial injuries. Medical Hypotheses 63(3): 442-445.
- Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, et al. (2011) Myocardial Ischemia in the Absence of Obstructive Coronary Artery Disease in Systemic Lupus Erythematosus. JACC Cardiovascular Imaging 4(1): 27-33.
- Sandhu VK, Wei J, Thomson LEJ, Berman DS, Schapira J, et al. (2020) A Five-Year Follow up of Coronary Microvascular Dysfunction and Coronary Artery Disease in SLE: Results from a Community-Based Lupus Cohort. Arthritis Care Res (Hoboken) 72(7): 882-887.
- Muller R (2021) Systemic toxicity of chloroquine and hydroxychloroquine: prevalence, mechanisms, risk factors, prognostic and screening possibilities. Rheumatol Int 41(7): 1189-1202.
- Joyce E, Fabre A, Mahon N (2012) Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care 2(1): 77-83.
- Yogasundaram H, Putko BN, Tien J, Paterson DI, Cujec B, et al. (2014) Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol 30(12): 1706-1715.
Citation: Spigno FD, Cacciola G, Halasz G, Aschieri D (2022) Myocardial Involvement in Systemic Lupus Erythematosus: Not only A Problem of Epicardial Coronary Artery. Curr Res Emerg Med 2: 1033