Journal of Mineral and Material Science
[ ISSN : 2833-3616 ]
Avocado Pear (Persea Americana) Leaves Extract as Corrosion Inhibitors of Medium Carbon Steel in 1.0M Hcl Solution
Department of Metallurgical and Materials Engineering, University of Nigeria, Nigeria
Corresponding Authors
Keywords
Abstract
This research work carried out the study on avocado leaves extract as a corrosion inhibitor in an acidic medium using the coupon testing method. The inhibitor concentration was varied, ranging from 1, 2, 3, and 4ML at temperature of 35.6 °C in a thermostatic water bath. The experiment was carried out in different time intervals of 3 days, 6 days and 9 days. The weight loss was then calculated, and the graphs of corrosion rates and inhibitor efficiency were plotted. The microstructural integrity of the samples was revealed using an optical microscope and SEM/EDX analysis. It was observed that the inhibition efficiency increased with inhibitor concentration and inhibition occurred through absorption of the inhibitor on the medium carbon steel surface. The absorption of these inhibitors obeys the Langmuir adsorption isotherm.
Introduction
Corrosion is a natural process, which converts a refined metal to a more stable form, such as its oxide, hydroxide, or sulphide. It is the gradual destruction of materials (usually metals) by chemical reaction with their environment. Corrosion is an undesirable phenomenon, which destroys the luster and beauty of the materials and lessens their life. Since ancient times corrosion has affected not only the quality of daily lives of the people, but also their technical progress. Corrosion also causes property deterioration such as loss of malleability, electrical conductivity; optical reflectivity by metal, i.e. corrosion degrades useful properties of materials and structures which includes strength, appearance and permeability to liquids and gases. Corrosion process is a means of releasing the energy that was stored-up in metals during metal extraction from their ores. The metal because of its high energy is in an excited state; thus, the corrosion process is a means of going to the lower energy state (the combined state). Corrosion is an irreversible interfacial reaction of a material with its environment, which results in the consumption of the material. It is, therefore, the natural tendency of the elements of a material to return to their most thermodynamically stable state [1]. The term corrosion is also sometimes applied to the degradation of plastics, concrete and wood, but generally refers to metals. Metallic corrosion is the natural process of the metal going to its oxidized state [1]. The metal was corroded by exposure to corrosive atmosphere like moist air, salty water, refinery oil, various acids etc. The most widely used metal is iron (usually as steel). Corrosion of metals constitute a serious ecological challenge which affects the global industries today where steel is used in different engineering structures, basically for the manufacturing of equipment and construction which comes alongside with problems of great interest. Acid solutions are widely used in industry for several purposes, such as acid pickling, industrial acid cleaning, acid descaling and oil well acidizing. Because of the general aggressiveness of acid solutions, inhibitors are commonly used to reduce the corrosive attack on metallic materials [2]. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term “degradation” is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases. Many structural alloys corrode merely from exposure to moisture in the air, but the process can be strongly affected by exposure to certain substances. Although there are numerous options for controlling the corrosion of metals, like cathodic protection, material selection, design and process control (which involves temperature, pressure, flow rate, etc.); but the use of inhibitors is one of the most practical methods for the protection of mild steel against corrosion especially in acidic media [2].
Recently, due to increasing concerns about the environment and stricter environmental regulations, low inhibitor toxicity is an important requirement for practical applications of inhibitors. Consequently, the current focus in corrosion inhibitor research is to identify and develop new classes of non-toxic, environmentally friendly and inexpensive alternatives [3]. In addition, plant products are low-cost, readily available and renewable sources of materials for inhibition purposes [3]. In most industries whose facilities are constituted by metallic structures, the phenomenon of corrosion is invariably present [4]. This results in material and economic losses due to partial or total replacement of equipment and structures that are affected in the process of corrosion. All over the world, the term corrosion is seen as a threat to economic development. It is an unavoidable phenomenon both in developed and developing nations of the world [4]. Apart from economic implications, corrosion also has both social and health implications. It affects the safety and health of people either working in industries or living in nearby towns due to toxic products. Heavy metals released from corrosion leads to cancer in human beings and the destruction of eco-system. In Nigeria, for example, millions of Naira is being spent every year to control losses of corrosion in oil companies. Oil pipelines are repaired virtually every quarter of the year due to leakages. Also, foreign corrosion inhibitors are usually used to reduce the effect of corrosion in the oil pipelines. This regular maintenance has led to the loss of a huge amount of money to companies. Meanwhile, the use of plant extract as corrosion inhibitors constitutes one of the most economical ways to mitigate the corrosion rate, protect metal surfaces against corrosion and preserve industrial facilities [5,6]. Hence, there is the need to find solutions locally to prevent economic waste.
Corrosion occurs when protective mechanisms have been overlooked, break down, or have been exhausted, leaving the metal vulnerable to attack. Corrosion of metals is of fundamental academic and industrial concern that has received considerable attention over the years. Corrosion of metals remains a global scientific problem as it affects the metallurgical, chemical, food processing and oil industries. Aviation, for instance, is a capital-intensive industry in which the imperatives are flight safety, the protection of investment and uninterrupted operation of aircraft over a long design life. In automobile manufacture, the design life is less but retail sales potential through positive customer perception is very important. Food handling introduces aspects of public health, biological contributions to corrosion problems, and the mass production of food cans that are low-value corrosion-resistant artefacts. Many failures due to the use of metallic structures in contact with aqueous and non-aqueous media have been reported because of the presence of corrosion in building construction [7]. An inhibitor is a substance which when added to acid solutions minimizes the loss of metal, reduces the extent of hydrogen embrittlement, protects the metal against pitting, reduces over pickling and acid fumes resulting from excessive reaction between the acid and basic metals and reduces acid consumption. Corrosion inhibitors are substances which when added in small quantity to corrosive environment lower the corrosion rate. They reduce corrosion by either acting as a barrier by forming an adsorbed layer or retarding the cathodic and/or anodic process [7]. National Association of corrosion engineering defines inhibitor as a substance, which retards corrosion when added to an environment even in small concentration [8]. Corrosion inhibitors [9] cause any corrosion retardation process or the reduction in the oxidation rate of the metal by addition of a chemical compound to the system. Inhibitors are often easy to apply without causing any significant disruption to the process. The use of corrosion inhibitors is one of the best methods of combating corrosion. Generally, three of the four components of a corrosion cell (anode, cathode, electrolyte and electronic conductor) may be affected by corrosion inhibitors in order to reduce corrosion.
However, there are several factors to be considered when choosing an inhibitor. Cost: the cost of the inhibitor to be used should not be high. Eco-friendliness: the inhibitor should be environmental friendly. Availability of the inhibitor determines the selection of it. Toxicity: the inhibitor should be non - toxic. Plant extracts offer several advantages over traditional inhibitors such as chromates, nitrites, benzoates, phosphates, amines, ammonia etc. Natural products of plants exhibit high surface activity, so the trend of using them has become increasingly important in recent years. Plant extracts are environmentally friendly, biodegradable, non-toxic, easily available and of potentially low cost. About 100 plant materials have been studied as corrosion inhibitors for mild steel, aluminum, zinc, copper and magnesium. Materials from all different parts of the plant viz., leaves, bark, nuts, flowers, fruits, fruit peels, essential oil, pulpy vegetable matter and seeds have been studied as corrosion inhibitors. The nitrogen heterocycles, tannins, oxygen-containing heterocycles like flavones, coumarins and flavonoids are reported to inhibit corrosion. Use of plant extracts offers higher efficiency because corrosion inhibition is fortified through synergism [10]. Plant extract is low-cost and eco-friendly, and can be obtained through simple extraction process [11]. The key advantage of using plant extract as the corrosion inhibitor is due to both economic and environmental profits. It has no hazardous effect on human health and eco-system. Some plant extracts have been studied as effective corrosion inhibitors of metal or alloys in HCl, HNO3 and H2 SO4 solutions and are found to be effective. Organic inhibitors have been the most widely used in petroleum refining processes because of their ability to form a protective layer on the metal surface in media with high hydrocarbon contents. At present, there are a number of organic inhibitors belonging to different chemical families located in foreign countries of the world and some of them are equally found in Nigeria [4]. In the world over, there is a growing trend towards using natural products called as non-toxic compounds or green inhibitors as corrosion inhibitors. A number of them such as Nimtree or Indian Lilac (Azadirachta indica) leaves found in India, Nepal, Pakistan, Bangladesh, Sri Lanka and Maldibes; Curry Tree (Murraya koenigii) leaves found in India; Pinwheel flower (Ervatamia coronaria) leaves found in Crape Jasmine in east India; Wasteland Weed (Purpurea) leaves found in India and Sri Lanka, Olive (Olea europaea) leaves found in Meaiterian Basin from Portugal to the Levant, Arabian Penisula, Asia and China [12,13], have been reported to inhibit the corrosion of metals in acidic media. Most of these naturally occurring plants are safe and can be extracted by simple procedure. In the same vein, some locally available plant leaves such as Pineapple (Ananascomosus) leaves, Ugu (Telfaira occidentalis) leaves, Pawpaw (Carica papaya) leaves, Bamboo tree (Bambusa vulgaris) leaves, Palm Tree or African Oil Palm Tree (Elaeis guineensis) leaves, Cassava (Manihot esculenta) leaves and Avocado pear (Persian Americana) leaves are also good green leave corrosion inhibitors. They are found in tropical regions of the world including Africa and Nigeria. Out of these locally available plants, it is found that Avocado pear (Persea Americana) leaves are most common in Nsukka area, in Enugu State of Nigeria. This was one of the factors in this research that led to using avocado pear leave as corrosion inhibitor of medium carbon steel in acidic medium. Avocado pear (Persian Americana) leave extracts are normally composed of complex organic species such as tannins, alkaloids and nitrogen bases, carbohydrates, amino acids and proteins as well as hydrolysis products. These organic compounds contain polar functions with N, S, and O atoms as well as conjugated double bonds or aromatic rings, which are the major adsorption centers [14]. The extract from these leaves are a mixture of various components, which results in the complex inhibitive
mechanism. However, it is rather difficult to determine the components present in these extract as they create their relatively high ability to inhibit corrosion. Thus, testing the inhibition potential of major components using available pure compounds could be an alternative to study the corrosion inhibition properties of these leave extracts [15]. Hence, the researcher is concerned with the major compounds available in the avocado pear leaves and compares their inhibitive properties in acidic medium using medium carbon steel. Medium-carbon steel is a metal having a wide range of engineering applications due to its good properties. It is widely used in structural applications such as load-bearing application, crankshaft, bolts, gears, heavy-duty machinery, mining equipment, cranes, springs, locomotive tyres, large forging dies, wire ropes, hammers, pipes and snaps for riveters [16]. This research work was performed using the Avocado pear (Persian Americana) leave as corrosion inhibitors of medium carbon steel in 1.0M Hydrochloric acid solution (HCl(aq)).
Materials and Methodology
Material procurement
The Medium Carbon Steel material was obtained from Steel Company Limited in Ogba, Lagos state in the form of ribbed bars of about 12mm in diameter. The chemical composition as supplied by the manufacturer is given in (Table 1). 1.0MHCl was prepared in the Laboratory of Pure and Industrial Chemistry, University of Nigeria, Nsukka and was used as the corrosive environment for this research work. Other materials used in the course of this research work include: Persian Americana, Electrochemical Analyzer, Water Bath, Grinding Desk, Silicon carbide abrasive papers (p150-p1200 grades), Desiccators, Set of Beakers, Polyester and Toast cup, Masking tape, copper wire, syringe and Electronic weighing balance.
Method
Preparation of corrosion coupons:
The 12mm diameter ribbed steel bar was machined in a Centre lathe to remove the ribbed surface into 10mm diameter in other to prevent crevice corrosion [17]. The steel bars were cut into suitable dimensions of 10mm by 10mm using a hacksaw. The material was smoothened using file, followed by grinding using Silicon Carbide paper of different grits (p150-p1200) and polishing in order to remove the gross scratches introduced by the cutting tool from the coupon surfaces [18]. After grinding and polishing processes were performed, the coupon was set for the experiment.
Preparation of inhibitor solutions:
100ml of 1.0M HCl was measured into twelve different 200ml beakers. The solution extract from the Persea Americana in different concentrations of 1, 2, 3, and 4ml, was separately measured using syringe into twelve beakers with the concentrations and quantities of the extract and acid (1.0M HCl). This formed the set of experiments making it twelve beakers. The corrosive medium contains the potential inhibitor solution extracts in the twelve beakers while the extra beakers containing only the acid served as the control.
Procedures for the Preparation of Aqueous Extract Avocado Pear Leaves:
Fresh Avocado Pear Leaves were obtained from Amukwa in Nsukka Local Government Area of Enugu State, Nigeria and were dried at room temperature. Thereafter, the dried leaves
Table 1: Chemical composition of medium carbon steel.
|
Elements |
Chemical Formula |
Weight in Percentage (wt%) |
|
Carbon |
C |
0.337 |
|
Silicon |
Si |
0.163 |
|
Sulphur |
S |
0.0464 |
|
Phosphorus |
P |
0.053 |
|
Manganese |
Mn |
0.813 |
|
Nickel |
Ni |
0.102 |
|
Chromium |
Cr |
0.178 |
|
Molybdenum |
Mo |
0.018 |
|
Vanadium |
V |
0.0046 |
|
Copper |
Cu |
0.36 |
|
Tungsten |
W |
0.0021 |
|
Iron |
Fe |
Balance
|
were pulverized. 500g of the plant material were macerated with 2.0L of 100% methanol (MeOH) and were extracted at room temperature for 48hrs with agitation processes. The resulting methanol was filtered and the filtrate was concentrated in vacuum at 40 °C in a rotary evaporator to obtain the aqueous extracts [19]. The percentage yield of the extract in the methanol was 60%.
Corrosion test
Weight loss method:
The initial weight of the sample was taken using the Analytical Weighing Balance. The Medium Carbon Steel samples were suspended in the solution with a thread. The experiments were performed at a temperature of 35.6 °C for 9days. The readings were recorded at 3 days interval and the weight loss was calculated.
Electrochemical test
The corrosion test was conducted with the use of Electrochemical Tester Model: CHI604E. The tests were conducted in accordance with ASTM G199-09 (2014 Standard Guide for Electrochemical Measurement). An electrochemical cell containing the potential inhibitor solutions was used as the electrolyte, consisting of three electrodes: the working electrode (sample), counter electrode (graphite rod) and silver/silver chloride electrode, which was used as a reference electrode (Ag/AgCl). The tests were performed from -1.0V to +1.0V. The Open Circuit Tests were allowed to run for 3600 seconds while the Tafel tests were allowed to run for the same duration in seconds after which the graphs were plotted.
Chemical composition of the medium carbon steel
The coupons have a percentage nominal (wt.%) composition as shown in (Table 1): Chemical Composition of the Medium Carbon Steel as received from the Steel Company Limited, Ogba, Lagos State.
Qualitative phytochemical analysis of the extracts
Qualitative phytochemical analysis of the extracts was done to determine the presence of secondary metabolites present according to standard procedure [20]. Test for Flavonoids, Tannins, Alkaloids, Saponins, and Glycosides were carried out. Presence of flavonoids was confirmed by yellow colouration which disappeared on the addition of H2 SO4 ; A blue-black colouration was taken as evidence for the presence of tannins; The presence of alkaloids was confirmed by the reddish-brown colouration of the solution; Formation of froth that persist indicates the presence of saponins; presence of brown ring at the interface indicates the presence of deoxy sugar characteristic of a glycoside.
Results and Discussion
Characterization of Medium Carbon Steel The metallographic analysis was performed using Optical Microscopy at Material Science Lab, University of Nigeria Nsukka, while the SEM and EDX experiments were performed in South Africa. From the micrograph of plate 1 above, it can be seen that the parent material contains ferrites and cementite. The ferrites are more compared to cementite, which shows that cementite will corrode first before the ferrites (Figures 1-4).
Discussion of the SEM/EDX
The image of parent material (coupon sample) of an optical microscope, SEM and EDS were viewed with x10 magnification and it can be observed that the two magnifications with respect to EDS for parent material sample shows that iron is the major element. This result shows that carbon is protected whereas iron is degraded. Also, the image for the SEM/EDS for Avocado pear of 4ml concentration shows that the extract acts as anodic protection, which means that the iron is protected. The quantity of iron in spectrum 7 of plate 4 is 41.1wt% and that of carbon is 16.5wt%. Based on all these values stated, it can be deduced that due to the quantity of iron present in avocado pear leaves, it can act as an anodic protection
Figure 1: X10 magnification using an optical microscope
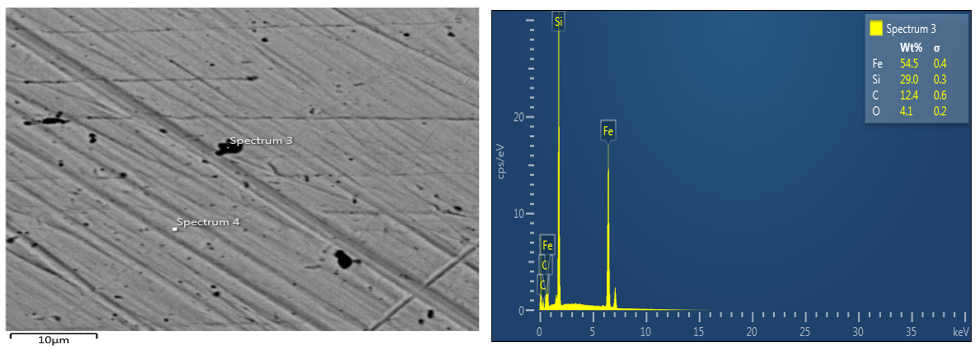 Figure 2: SEM/EDS for parent material.
Figure 2: SEM/EDS for parent material.
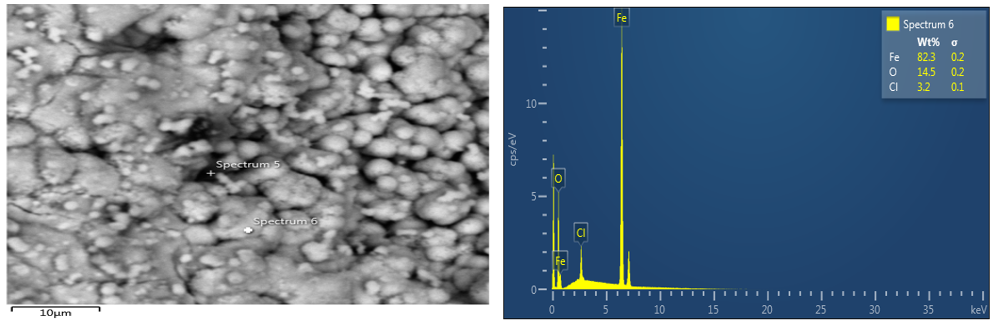 Figure 3: SEM/EDS for control after 9 days without inhibitors.
Figure 3: SEM/EDS for control after 9 days without inhibitors.
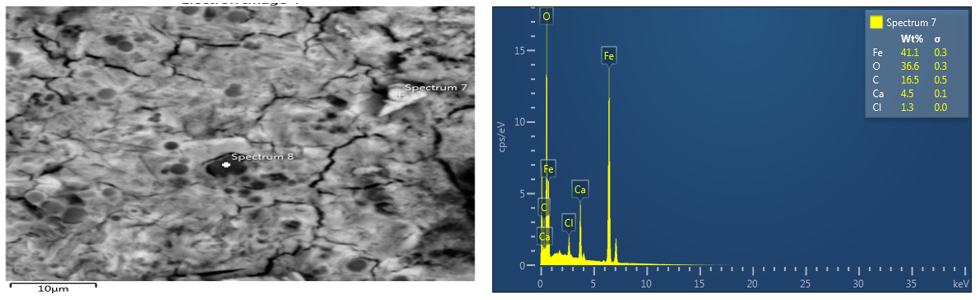 Figure 4: SEM/EDS for coupon immersed in Avocado pear leaves extract for 9days on 4ml.
Figure 4: SEM/EDS for coupon immersed in Avocado pear leaves extract for 9days on 4ml.
magnifications with respect to EDS for parent material sample shows that iron is the major element. This result shows that carbon is protected whereas iron is degraded. Also, the image for the SEM/EDS for Avocado pear of 4ml concentration shows that the extract acts as anodic protection, which means that the iron is protected. The quantity of iron in spectrum 7 of plate 4 is 41.1wt% and that of carbon is 16.5wt%. Based on all these values stated, it can be deduced that due to the quantity of iron present in avocado pear leaves, it can act as an anodic protection.
Graphs and Discussion
Visual observation
The metal samples used for the research work were critically observed and discovered that the initial colour of the metal changed from the steel colour to dull colour which, indicted some experimental processes had taken place . This translate the colour when no inhibitor was used the materials served as control sample. There was some precipitations occurred on the surface with yellow colouration when the samples were immersed in a solution with inhibitors and the degree of the yellow colour increased with increase in inhibitor concentration. This occurrence was due to the formation of films that hinders the anodic dissolution of the sample.
Graph of corrosion rate
From the results obtained and the graphs above on the corrosion rate of the steel coupons against exposure time. Control sample graph shows that the corrosion rate increases with an increase in exposure time (Figures 5 & 6). However, the corrosion rate decreases with increase in inhibitor concentration over time. This increase in corrosion rate with an increase in exposure time is because of an increase in exposure time brings about an increase in chemical reaction [21]. Therefore, the corrosion rate decreases with increase in inhibitor concentration. This is due to the absorption of an inhibitor molecule on the surface of the steel. This behaviour proves that the adsorption of the inhibitors on the medium carbon steel surface occurs through physical adsorption [22]. As seen from the tables and graphs above, the Avocado Pear leaf extract was observed to have good inhibited corrosion mechanism.
Graph of inhibition efficiency for the inhibitors
From the results obtained above, it was observed that the inhibition efficiency of the inhibitor increased with increase in the concentration of the inhibitors throughout the time of exposure (Figure 7). The general increase in inhibitor efficiency with concentration was credited to the adsorption of inhibitor molecules on the surface of the medium carbon steel. This is in accordance with the research work carried out by Valek [23] and Nwoke [24].
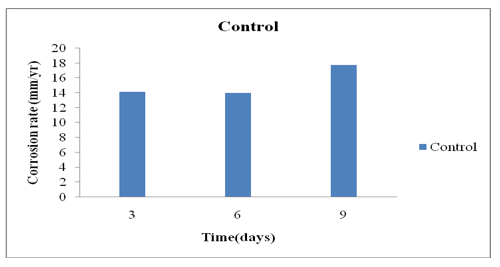
Figure 5: Corrosion rate against Time for Control.
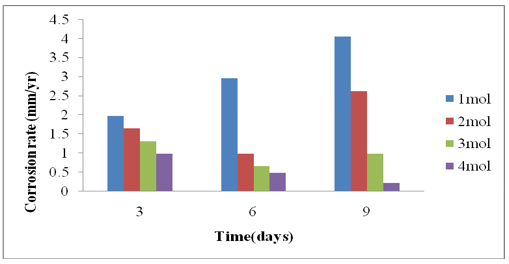
Figure 6: corrosion rate against Time for Avocado Pear
Graph of Open Circuit Potential Time (OCPT) and Tafel Plots for Avocado Pear Leaves Extract
(Figures 8-15)
Discussion of the OCPT and Tafel Plot from Figures 8 To Figures 17
Many sources explain the concept of open circuit potential time (OCPT) as voltage measurement when no current is flowing through the cell; hence, it is also the electrochemical potential of the solution. Therefore, OCPT is thermodynamic parameters, which guide us about the thermodynamic tendency of that metallic material to participate in the electrochemical neighbouring medium. Based on the above facts, it can be summarily stated that the higher the corrosion potential, the lower the corrosion rate and the lower the corrosion potential, the higher the corrosion rate. Also, avocado pear leaf extract exhibit passivation as a good inhibitor. Furthermore, Tafel is an electrochemical
kinetics relating to the rate of an electrochemical reaction to the overpotential. Tafel plots is a very important measurement of corrosion and it is one of the polarization method used to compare with weight loss but Tafel curves are not the same as those measured with weight loss method. However, the corrosion rate is proportional to corrosion density which shows that the higher the corrosion density, the higher the corrosion rate and the lower the corrosion density, the lower the corrosion rate. See (Figures14-17). Finally, from the graph of Tafel plots, the following corrosion rates were calculated in mil/year (mm/ yr.) for avocado pear leaves extract. Therefore, the following corrosion rate were evaluated for avocado pear leaves as; 1ml=5.545x104x104, 2ml=24.05, 3ml=33.92, and 4ml=2.904 x104x104 Mil/year. Following, data given above shows that 2ml for Avocado pear leaves extracts has the lowest corrosion rate among other values stated and it is 24.05 Mil/year.
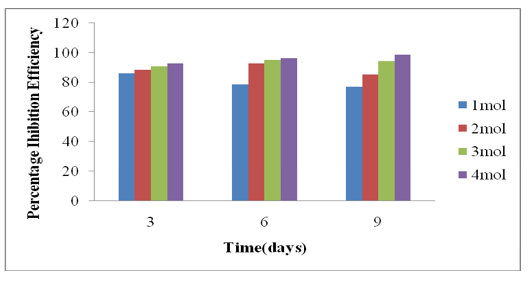
Figure 7: Percentage Inhibition efficiency against Time for Avocado pear.
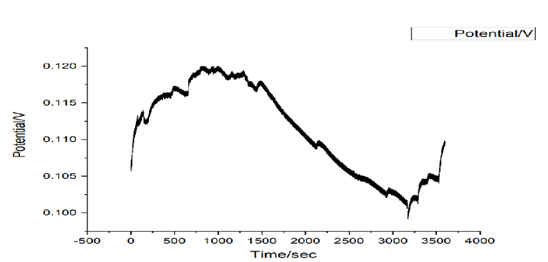
Figure 8: Potential against time for 1ml (Avocado pear leaves).
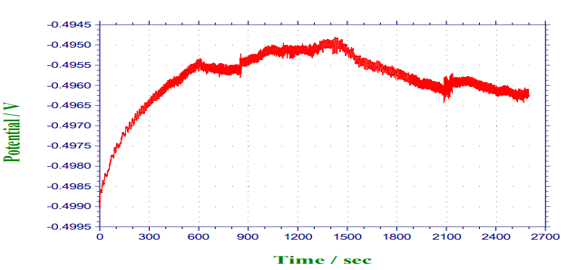
Figure 9: Potential against time for 2ml (Avocado pear leaves).
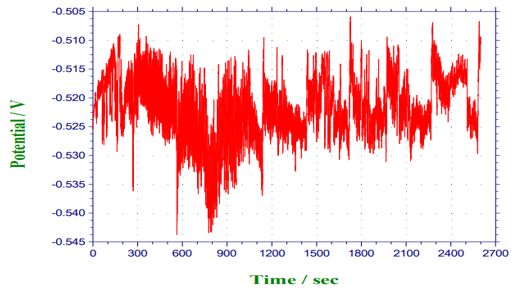
Figure 10: Potential against time for 3ml (Avocado pear leaves).
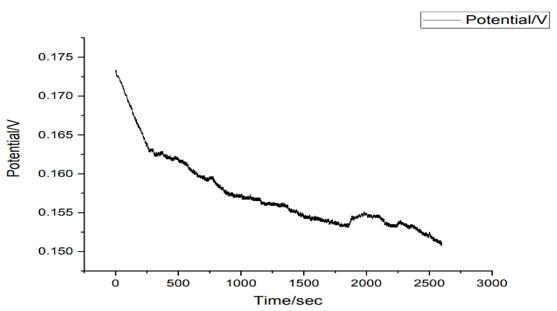
Figure 11: Potential against time for 4ml (Avocado pear leaves).
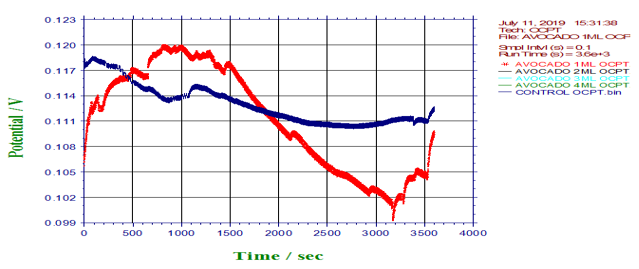
Figure 12: Potential against time for control, 1ml, 2ml, 3ml, and 4ml (Avocado pear leaves).
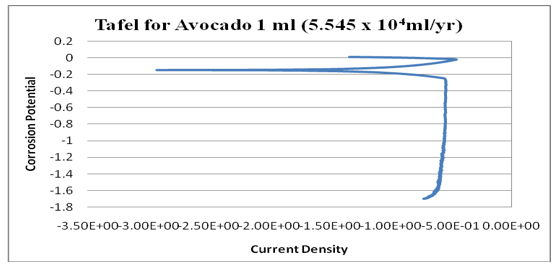
Figure 13: log (current/A) against potential/V for 1ml (Avocado).
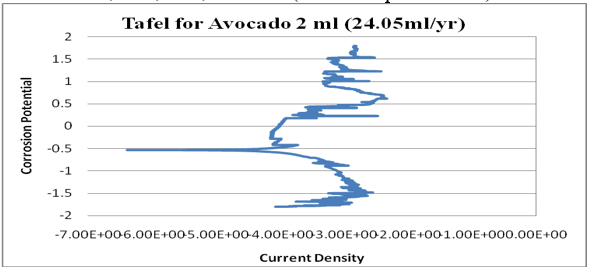
Figure 14: Graph of log (current/A) against potential/V for 1ml (Avocado).
Figure 15: Graph of log (current/A) against potential/V for 2ml.
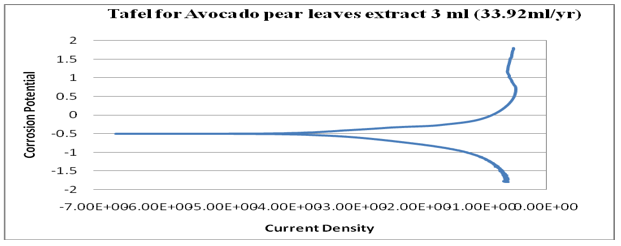
Figure 16: Graph of log (current/A) against potential/V for 3ml (Avocado).
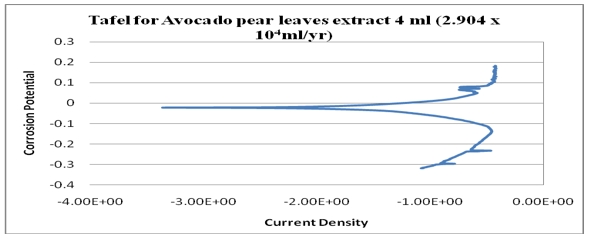
Figure 17: Graph of log (current/A) against potential/V for 4ml.
Conclusion
This research work performed was on the study on avocado leaves extract as a corrosion inhibitor in an acidic medium with the use of coupon testing method. The concentration of the inhibitor used were taken from 1, 2, 3 and 4ML. These processes were done at room temperature in a thermostatic water bath. The experiments were performed as designed for different time intervals of 3 days, 6 days and 9 days. The weight loss calculated gave the corrosion rate while the graphs plotted was corrosion rates against inhibitor efficiency. The SEM/EDXs were used to determine the morphologies of the materials used for the corrosion tests before and after the experiments. The microstructural of the samples were determined which, in turn structures of the materials used during the experiments it was discovered that the inhibition efficiency increased with inhibitor concentration. The inhibition occurred through absorption of the inhibitor on the medium carbon steel surface. The absorption of these inhibitors was in line with the Langmuir adsorption isotherm principle. This research work was based on the study of avocado pear leaves as corrosion inhibitors of medium carbon steel in 1.0M of HCl solution. The observations from the research indicated that Medium Carbon Steel was affected by corrosion and hence, might fail with time. Due to these observations, corrosion prevention techniques are necessary to prolong the life span of the Medium Carbon steel. Some corrosion monitoring techniques could be deployed which includes the use of inhibitors, surface treatments, coatings, cathodic protection and anodic protection, material selection and design. It could also be indicated that the use of inhibitors for corrosion control was necessary due to their potent and efficiency over others (i.e. availability, non-toxic and eco-friendly).
The Avocado pear leaves are eco-friendly, non-toxic, and are commonly found in Nsukka, Enugu State of Nigeria; and it was observed that they have good inhibit corrosion on the Medium Carbon steel. The conclusion from this work indicated that the Avocado pear leaves extract inhibits corrosion. Its inhibition efficiency was 98.7654 and its corrosion rate was 0.2194mm/yr. The inhibition efficiency of the avocado pear leaves extract increased with an increase in the inhibition concentration. Corrosion inhibition occurs through absorption of the inhibitors on the surface of the Medium Carbon steel. The adsorption of the inhibitor obeys the Langmuir adsorption isotherm.
References
1. Shrier LL, Jarman RA, Burstein GT (Eds.) Corrosion. Volume I: Metal/Environment Reactions.
5. Sastri VS (2011) Green corrosion inhibitors. Theory and Practice. John Wiley & Sons: New York. pp. 5-10.
6. Sastri VS (1998) Corrosion inhibitors. Principles and Applications. John Wiley & Sons: New York.
7. Uppal MM, Bhatia SC (2001) Engineering Chemistry. (6th edn), Khana Publishers, Delhi, India.
8. (1965) NACE International Corrosion Conference and Expo, USA. 10149: pp. 1-2.
9. Hackerman N, Hurd RM (1962) corrosion inhibition and molecular structure, first international congress on metallic corrosion. Butterworths, London. pp. 166-170.
11. Xianghong LI, Shuduan D, Hui FU, Xiaoguang XI (2014) Corrosion science. Degradation of Materials and its Control 78(1): 29.
16. Aigbodion VS (2018) Theory and Principle of Engineering Alloy. MME Dept, Faculty of Engineering, University of Nigeria, Nsuuka, Nigeria.
18. Colwell RL (2005) Metallographic Analysis. In: Baboian R (Ed.) Corrosion Tests and Standards: Application and Interpretation (2nd edn) West Conshohocken PA: ASTM International, USA.
19. Sankara Narayanan T (2018) Corrosion and corrosion preventive methods preventive methods. National Metallurgical Laboratory Madras Centre, Chennai. pp. 156-157,
20. Harborne JB (1984) Phytochemical methods. A guide to modern techniques of plant analysis (2nd edn), Chapman and Hall, New York. p. 288.
21. Singl A, Ahamad I, Singl DK, Quraishi MA (2013) The effect of environmental benign fruit extract of Shahjan (Moringa oleifera) on the corrosion inhibition of mild steel in hydrochloric acid solution. Chemical Engineering Communications 15: 1087-1097.
23. Valek L, Martinez S, Mater L (2007) Anti-corrosion properties of areca palm leaf extract on aluminum in 0.5 M HCl environment. South African Journal of Chemistry 71: 148-151.
Citation: Omah AD, Obiorah HA, Ocheri C*, Offor PO, Ezema Ike Eze IC, and Daniel Mpkume CC. Avocado Pear (Persea Americana) Leaves Extract as Corrosion Inhibitors of Medium Carbon Steel in 1.0M Hcl Solution. J Miner Sci Materials. 2020; 1(1): 1004.