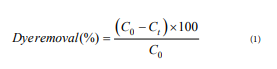Journal of Mineral and Material Science
[ ISSN : 2833-3616 ]
Comparison between Divergent and Convergent Synthetic Routes of Functionalized Silica by Amine- Terminated Dendritic Materials
Textile Engineering Department, Amirkabir University of Technology (Polytechnic Tehran), Tehran, Iran
Corresponding Authors
Keywords
Abstract
Comparison between divergent and convergent synthetic routes of functionalized silica by amine-terminated dendritic materials for adsorption of anionic dye is the purpose of this article. Pristine silica has hydroxyl groups and low affinity for adsorption of anionic dyes. As a result, functionalization of silica by dendritic structure was enhanced adsorption capacity with two ways. Positive amino terminal activated groups on functionalized silica increased its adsorption capacity with ionic adsorbent and the cavity between branches improved physical encapsulation. For this aim, different generation of amineterminated dendritic group were synthesized on pristine silica by two divergent and convergent methods. Techniques such as Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM) and EDX were utilized to characterize those synthetic routes. To optimize adsorption condition, some parameters like pH, adsorbent dosage, contact time and initial concentration were investigated by dye removal percentage of C.I Acid Red 1 as a model anionic dyes. The result reveals that in desire pH condition, adsorbent dosage and initial concentration, dye removal percentage was enhanced from zero for pristine silica to 94.8 and 90.6 % for functionalized silica by divergent and convergent methods, respectively. Furthermore, adsorption isotherm and kinetic were studied. The results reveal that the adsorption isotherm follow Freundlich isotherm and kinetic information fit pseudo-second order adsorption. Although, the divergent synthetic routs has greater removal percentage, the convergent method is an easier way to spend less time for synthetic methods.
Introduction
The presence of organic dye in water made it pollutant and caused series environmental problems [1]. Impurity release caused harmful disease and influenced on human health such as different concern [2]. Different chemical industrials made a huge of industrial wastewater [3]. Residue dyes from industry such as produce paper, textile, and manufacture dye were be danger for environment and diseased human health [3]. As a result, removals of these pollutants from wastewater absorb lots of attention. In recent years, some techniques were used for the removal of dye from aqueous solution such as degradation, oxidation, photocatalytic degradation and adsorption that among all of them, adsorption was more important [4]. Some adsorbents such as halloysite, graphene oxide, silica were regularly utilized via adsorption technique. Among various mineral adsorbents, silica particles are more available due to its suitable cost, abundant in many countries, high surface area, controllable pore size and high durability [5]. Silica particles due to its high surface area can be use in catalysis, separation and wastewater [3]. Silica is a mineral material that is abundant in the earth crust and is usually found in combination. Due to better availability of silicon oxide, the production process of this element at industrial scale were generally started by oxide production. The most important source of silicon is silicon oxide with the molecular formula of SiO2 , which is one of the most abundant materials in nature [6]. Silica is the only inorganic industrial polymer that is widely used as raw material and additives due to their exceptional properties. These materials are also stable at high temperature and resistant from oxidation and weathering [6]. Despite, novel characteristic of silica particles, they have some limitation. As a result, much attention has been paid to silica chemical functionalization to improve its physical and chemical properties [7]. The adsorption capacity and selectivity of silica depends on the type and number of functional groups in the mineral structure. Surface functionalization of silica changes properties and it can be uses in specific applications [8]. Silica is one of the most moisture adsorbent materials known due to its high water adsorption capacity (40% w.t) and easy recovery compared to zeolite. Its surface is also ready to modify by reaction and bonding with organic materials [6]. The bond between silica and the amine group prepares silica to selective and environmental adsorbent applications in order to control and acceleration the process [6]. Zhang et al were functionalized silica via ester and amine-terminated groups with amidoamine. They were functionalized silica and were obtained fourth generation of silica. According to SEM of these nanoparticle, size and shape of them were constant that shows high mechanical durability of silica nanoparticle. Adsorption capacity of heavy metal was determined and result shows that adsorption capacity was release from second to fourth generation [9].
Although lots of paper focus of various silica functionalization, functionalization of dendritic silica attract more attention due to its novel characteristics [10]. Recently, dendritic polymers has been used to increase adsorption capacity. In this way, the functional groups of the materials are more accessible and can be achieved with the purpose of removing pollutants from the wastewater. In this method, dendritic structure was synthesized on silica core by using methyl-acrylate and ethylene diamine at specific temperature and periods of time [7]. The best method for removing materials is modify them with dendritic polymers because of the advantage of increasing the amine end group at the end of the spherical and symmetrical dendritic structures, which results in improved hydrophilicity, biocompatibility, and in general, improved surface properties of the adsorbent. Moreover, utilization of dendritic nanoadsorbents economized money [11]. Dendritic polymer has ability to grow around a central core and, based on this, have the capability of retaining any external molecule. Among these compounds, the dendrimer with the amine terminated group are considered. The convergent synthesis method is known by Frechet, which in this method the lateral branch connected to the end group is synthesized and finally connected to the core by completing the synthesis [12]. In divergent synthesis method, a multi-core nucleus molecule, such as ethylene diamine, is started, and with Michael’s reaction, there are four lateral branches on the surface of the nucleus, which is located on each side of the nitrogen. Then, by adding ethylene diamine, terminated of the four lateral branches be amine, and by repeating these steps, desired generation can reach. It should be noted that the number of branches in each generation is twice that of its predecessor, and in order to prevent the generation of defects in higher generations and disorders. In this method, more polymer content has been obtained, but less pure than the previous one, and this method has been taken into consideration in the industry [13]. The aim of this article is functionalization of silica particle by amine-terminated dendritic materials via two different synthetic routes. The functionalized silica in both divergent and convergent methods were investigated for removal of Acid Red 1 as an acidic model dyes. Adsorption isotherm and kinetic of this anionic dye were investigated, too.
Experimental
Material
Silica, (60.08 Molecular Weight) was purchased from LOBA Chemie Co., India. Acid Red 1 was purchased from Ciba Ltd. Second equivalent generation amidoimine polymer was obtain from Delta Inovative company, Poland. The pH adjusted with HCl and NaOH 0.1N. Aminepropyltriethoxysilane (APTES), methyl acrylate (MA), ethanol, and ethylene diamine (EDA) were purchased from Aldrich. All chemicals were laboratory reagent grade. The chemical structure and dye specifications are presented in Figure 1 and Table 1, respectively
Table 1: Anionic dye characteristics.
|
Dye |
CAS No. |
Chemical Formula |
Molecular Weight |
Color Index No. |
Chemical Class |
|
C.I Acid Red 1 |
6-67-3734 |
C18H13N3Na2O8S2 |
509/42 |
118050 |
Mono azo |
Functionalization of Silica via divergent synthetic route
Functionalization of pristine silica with APTES:
In order to form amine groups, silica was stirred for 6 hours in APTES and toluene at 70 °C. After being separated by a Buchner funnel, it was washed several times with ethanol to neutralize. Then functionalized silica was dry in oven for 24 hours at 50 °C. The resulting product is called G0.
Functionalization of G0 with MA:
In next step, G0 stirred with methyl acrylate and methanol at 50 °C for 3 days. Then, silica washed with ethanol and THF after separation by the Buchner funnel to neutralize this step product. Neutralize product placed in oven at 50 °C for 24 hours. The product of this step is abbreviated by G0.5.
Functionalization of G0.5 with EDA:
In the final step, for the formation of amine groups, G0.5 with ethylene diamine and methanol was stirred at room temperature for 5 days. Then, silica washed with ethanol and THF after separation by the Buchner funnel. In the next step, these products were kept in the oven at 50 °C for 24 hours. The product of this step is called G1.0. The described process leads to the generation of dendrimer structure on the surface of silica. To produce a desirable generation, the process was repeated to reach second generation. Figure 2 shows the synthesis route of dendritic structure onto silica via divergent method.
Silica functionalization via convergent synthetic route
In this method, G0.5 and second equivalent generation amidoamine polymer were dissolved in methanol and stirred at room temperature for 5 days. Then, functionalized silica washed with ethanol after separation by the Buchner funnel. This product was dried in oven for 24 hours at 50 °C. The product of this step is called GD.
Adsorption test
The adsorption of functionalized silica was tested with preparation of 250mL AR1 solution (20mg/L) and added different mass of dried adsorbents. The pH of dye solution was adjusted to 3, 5 and 7. Solution at an ideal pH with different dye concentration of (20, 50, 75 and 100mg/L) was examined for discovering the effect of initial dye concentration. Also, solutions with different adsorbent dosages (0.2, 0.3, 0.4 and 0.5g/L) were analyzed at various time with a spectrophotometer (UNICO 2100) at maximum wavelength of AR1 which is 506nm. The dye removal studied by Equivalent (1), respectively
Where t, C0 and Ct are time, primary dye concentration and concentration at time t, respectively.
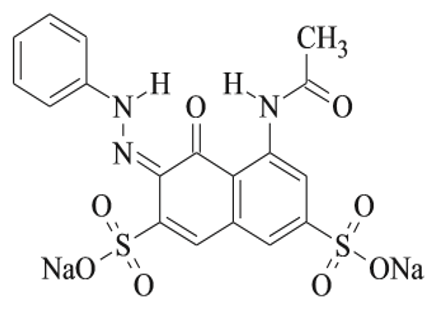
Figure 1: Chemical Structure of Acid Red 1.
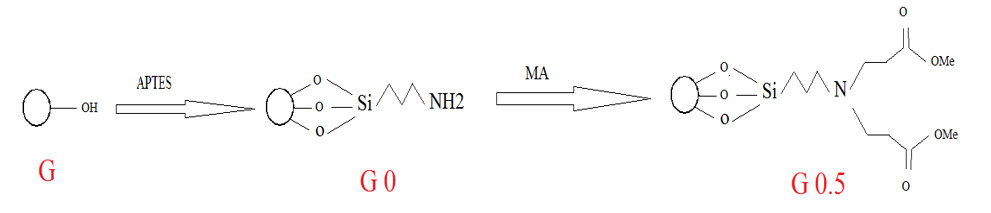
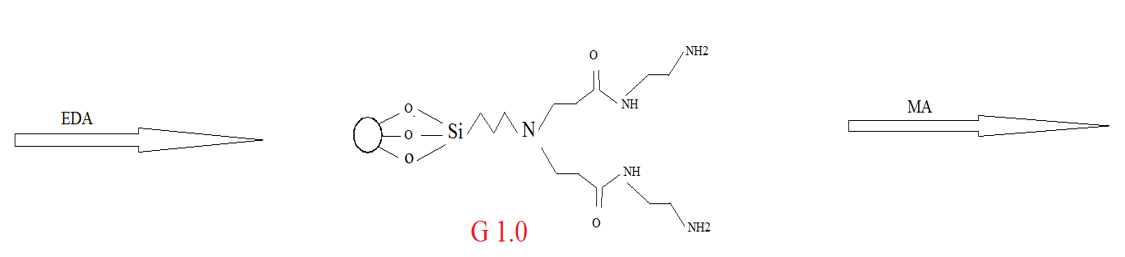
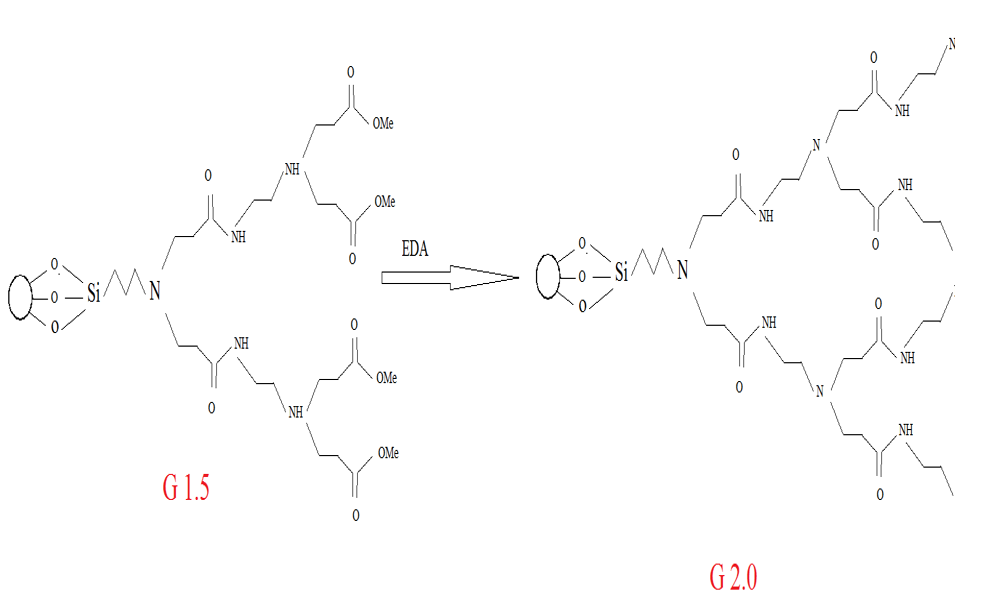
Figure 2: Synthetic routes of ester and amino-terminated dendrimers-like PAMAM polymer-grafted silica.
Where t, C0 and Ct are time, primary dye concentration and concentration at time t, respectively
Adsorption isotherm
In order to increase the removal percentage of anionic dye by adding functionalized silica, the effect of functionalized silica with dendritic structure on dye adsorption was investigated. The solution with 20-50-75-100mg/L concentrations was prepared. These solutions were prepared with a certain percentage of adsorbent and at room temperature for 60 minutes with a magnetic stirrer and at different sampling times. The adsorbent was separated by centrifuging samples at 5000rpm for 10 minutes at room temperature. The amount of adsorption of anionic dye before and after adsorption of the adsorbent was measured by spectrophotometer in the maximum wavelength of anionic dye.
Kinetics study
These experiments were performed by contacting a certain amount of adsorbent with dye solution at room temperature at period of times. Sampling was performed at predetermined time intervals and in predetermined volumes. Then, the samples were centrifuged for 10min at 5000rpm to separate the adsorbent from the solution. Finally, the absorbance was measured by spectrophotometer.
Characterization
d functionalized silica, the VEGA/TESCAN model of scanning electron microscopy and the EMI-tec gold model of gold coating layer was used. The type and percentage of the elements in expandable polymers were measured with using VEGA\TESCAN model and the EMI-tec Gold Layer Coating Machine by EDX method. In order to prove the functionalization of the silica chemical structure, utilized using the FTIR measurement was the Thermo-Nicolet Nexus 870 low-intensity spectrometer was used in the range (400-4000cm-1) with a resolution of 4cm-1 and a mean of 32 scanning FTIR. Spectral analysis of the samples was performed using OMNIC. For quantitative analysis, the intensity of all peaks were normalized to reference peak at 1103cm-1, which is Si-O-Si peak, to be have same intensity in all spectra [14].
Results
Study chemical structure of pristine and functionalized silica with FTIR
FTIR spectra of pristine silica and various generation of functionalized silica are shown in Figure 3. Strong peak at 3430cm-1 which was demonstrated presence of Si-OH groups on silica surface in all samples. All samples had strong peak at 1103cm-1 and weak peak at 799cm-1 which were demonstrated Si-O-Si groups. New peak at 2850-2950cm-1 in G0 relate to the symmetric and asymmetric CH2 peak, respectively. Peak at 1730cm-1 in G0.5 and G1.5 demonstrate to form carbonyle group. New peaks at 1550cm-1 and 1640cm1 in G1.0 and G2.0 indicate primary amine group and assign bending primary and secondary amine groups. Peak at 1500-1600cm-1 in GD show amide group of dendritic structure with convergent method [14]. These peaks show that dendritic structure with amine terminated groups on silica surface successfully were synthesized.
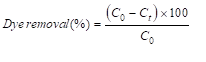
SEM
Surface structure and morphology of pristine silica and functionalized silica, were studied by SEM analysis that was shown in Figure 4. Each generation was decreased silica size while, the morphology was not changed that indicated mechanical durability of these particle. Probably, the decrease size of functionalized silica was due to separating
Table 2: Quantitative percentages of N/Si0
|
Sample |
N/Si |
|
G |
0.1233 |
|
G0 |
0.1434 |
|
G1 |
0.2719 |
|
GD
|
0.2372 |
agglomeration of silica particle. It seems that functionalized silica particle in G1.0 and GD samples toward G sample were more available that cause more reactivity and adsorption materials with negative charge.
EDX
Type and elements percentage of all particle were measured by EDX method. N to Si ratio was increased with increasing silica structure generation that shown growing positive dendritic structure on silica core as shown in Figure 5 & Table 2.
Adsorption study
Effect of pH
Dye adsorption was studied in different pH condition to optimize this important parameter on dye adsorption. In acidic pH, acid was released H+ and according to negative charge of dye and positive charge of amine terminated groups on functionalized silica
Table 3: Isotherm parameters for the adsorption of AR1.
|
Dye |
Langmuir |
Temkin |
Freundlich |
|||||||
|
AR1 |
Qm |
KL |
RL |
R2 |
B1 |
KT |
R2 |
KF |
N |
R2 |
|
833/3 |
91/743 |
0/00011-0/00055 |
0/93 |
84/3 |
4/49 |
0/99 |
81/85 |
1/22 |
0/98 |
|
dye removal was enhanced significantly as shown in Figure 6. Dye removal percentage was measured 90% in acidic and 3.2% in basic pH. According to result, acidic pH was provided suitable condition for removing anionic dye due to electrostatic attraction on functionalized adsorbent surface. The structure and form of dendrimer is different in variant pH that dendrimer structure is open in acidic pH and collapse in alkali pH.
Effect of adsorbent dosage
(Figure 7) reveals the effect of adsorbent dosage in optimum pH. According to result, acidic pH made suitable condition for anionic dye adsorption.
Effect of dye concentration
(Figure 8) is shown that dye removal decrease with increasing dye concentration. With increasing dye concentration, dye particle agglomeration was enhanced that this agglomeration was increasing molecular size that decrease occupation expecting on adsorbent holes. Dye removal percentages in 100ppm dye concentration is high confirmed high adsorption capacity of functionalized adsorbent.
Effect of generation of dendritic structure
Silica has negative charge due to its hydroxyl groups on the surface. After surface functionalization by dendritic polymer, amine terminated group with positive charge grow on its surface and increase dye removal. Also, with growing generation, host hole number for accepting dye molecules increase and enhance dye molecules occupation. Not only cationic ion at the functional group of dendritic structure increases, but also the cavity between branches enhanced. This result is shown in Figure 9. According to
Table 4: Kinetic parameters for the adsorption of AR1
|
Pseudo-Second-Order |
Pseudo-Second-Order |
Intraparticle Diffusion |
||||||||
|
C0 |
qe(exp) |
k1 |
R2 |
qe |
Kad2 |
h |
R2 |
C |
Kp |
R2 |
|
20 |
0.25996 |
0.0182 |
0.0066 |
63.69 |
2.74 |
11114.58 |
1 |
63.065 |
0.138 |
0.2087 |
|
50 |
0.1361 |
0.0405 |
0.0175 |
161.29 |
15.5 |
403224.1 |
1 |
158.38 |
0.645 |
0.9125 |
|
75 |
3.8124 |
0.126 |
0.136 |
238.09 |
10.5 |
595211.9 |
1 |
224.78 |
2.858 |
0.7863 |
|
100 |
0.9188 |
0.0196 |
0.0033 |
312.5 |
16.00 |
156250000 |
0.99 |
308.44 |
1.220 |
0.5127 |
de Gennes theory in dendritic structure after a certain generation cross linking between terminated groups create a dense shell and have deviance from ideal dendrimer that permeation is not possible and dye removal decrease [15].
Adsorption isotherm
Anionic dye equilibrium adsorption process on functionalized silica was studied. Data were analyzed by R2 value of each model. Comparison R2 values of three models according (Table 3 & Figure 10), show that R2 values of Temkin and Freundlich are highest. Due to the abundant amine terminal groups at the exterior and hollow dendritic box between cavities in exterior of branches, Freundlich model is better to follow with linear graph and 0/98R2 . Presence of empty regions between branches were conducted adsorption from one layer adsorption in Langmuir model to Freundlich model.
Adsorption kinetic
Adsorption kinetic is one of the adsorbent efficiency criterion. The fitted kinetic models to the experimental data demonstrated that the adsorption process followed the pseudo-second-order kinetic model shown in Figure 11. By pseudo-second-order kinetic model observed that increasing dye concentration causes kinetic constant increase and so equilibrium time decrease (Table 4).
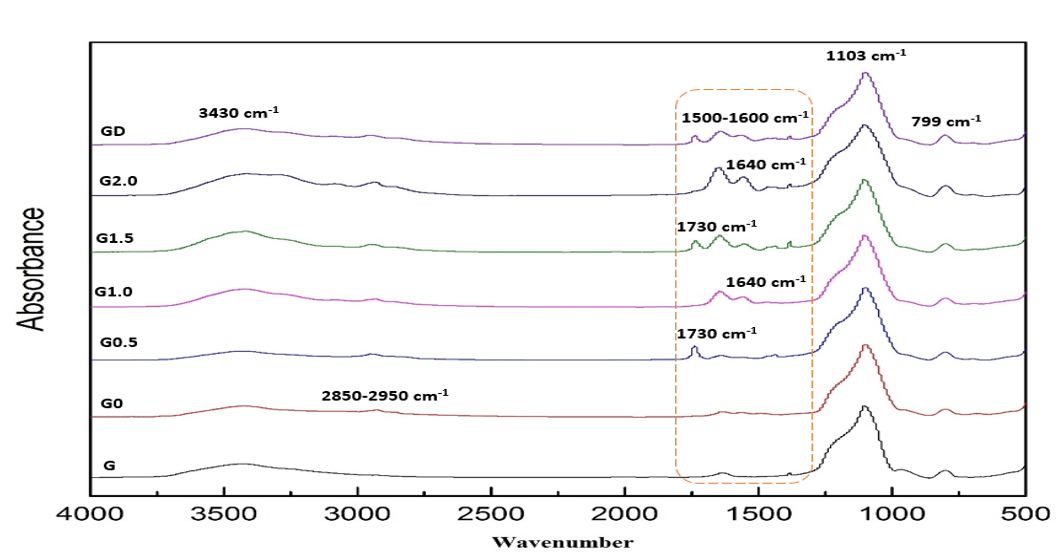
Figure 3: FTIR Spectra of G, G0, G0.5, G1.0, G1.5, G2.0 and GD.

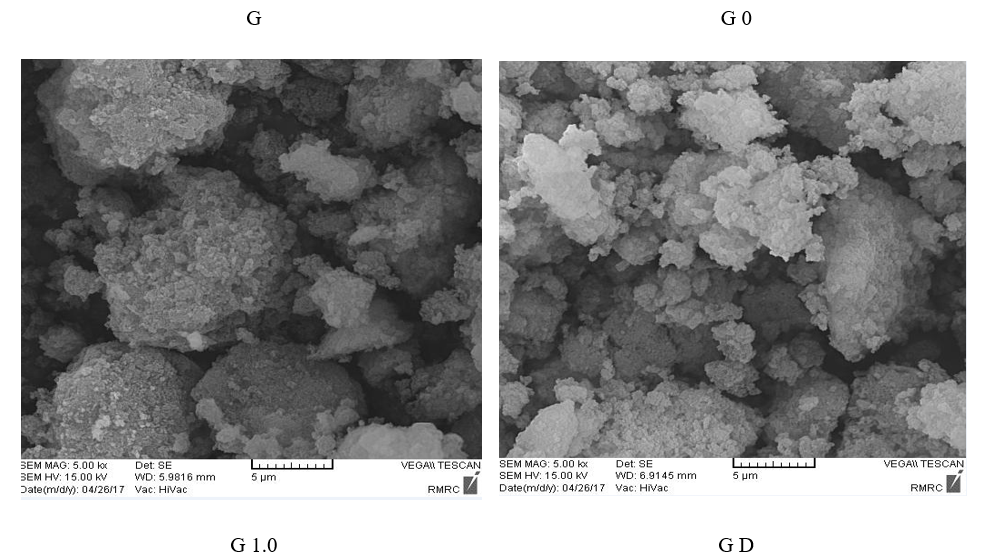 Figure 4: The SEM images of G, G0, G1.0 and GD.
Figure 4: The SEM images of G, G0, G1.0 and GD.
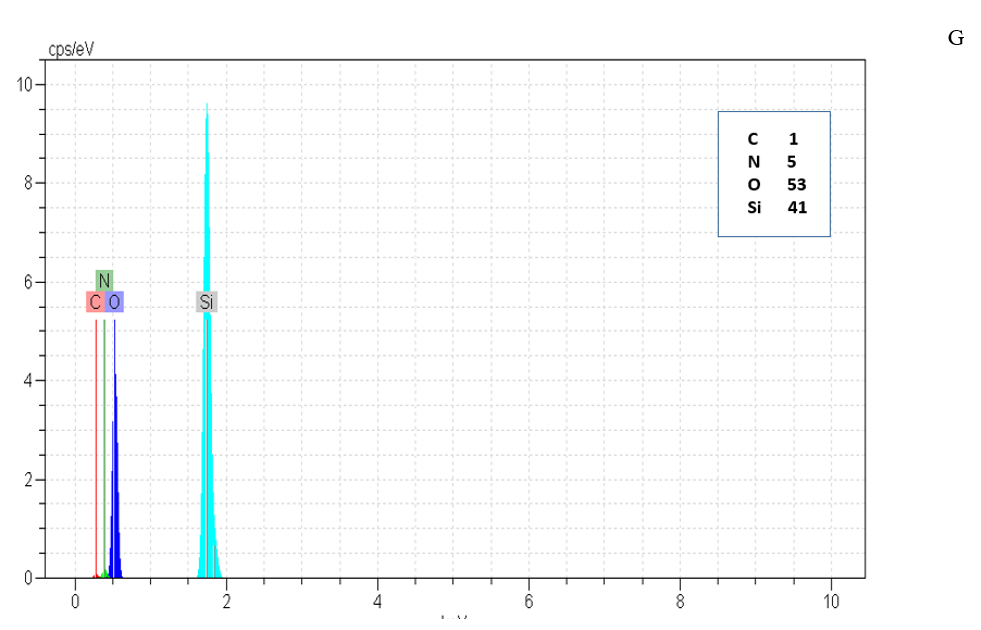
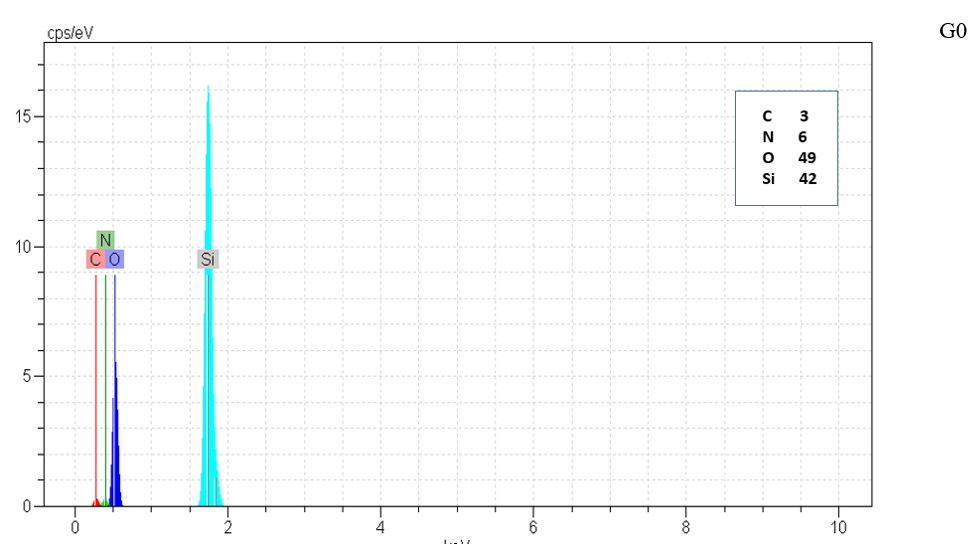
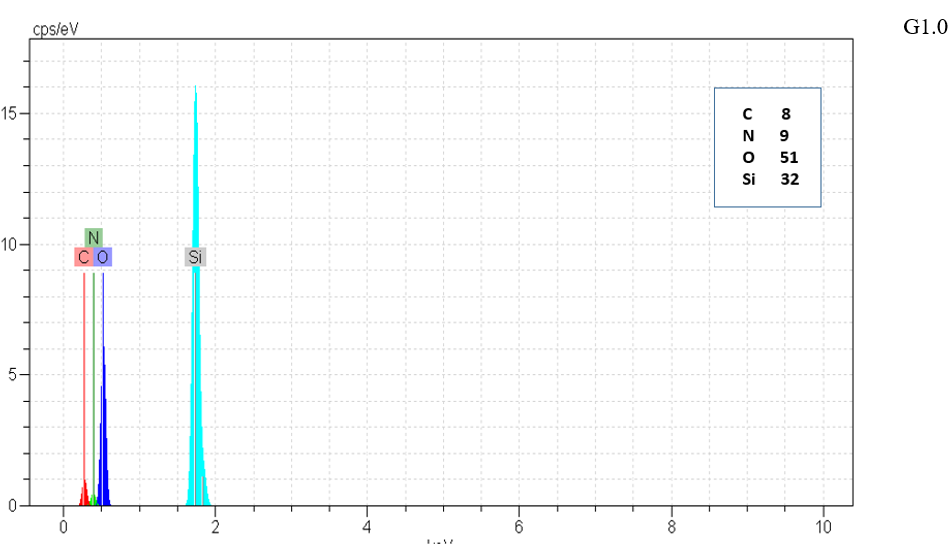
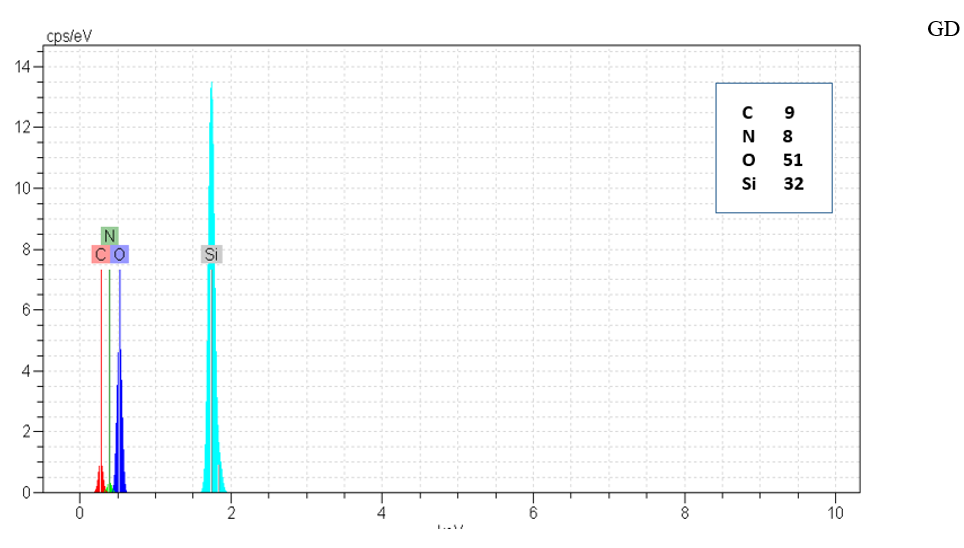 Figure 5: EDX of G, G0, G1.0 and GD.
Figure 5: EDX of G, G0, G1.0 and GD.
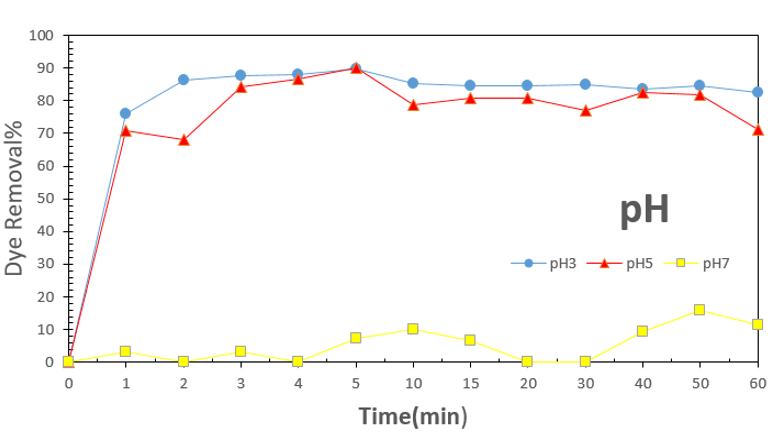
Figure 6: Effect of pH on dye adsorption.
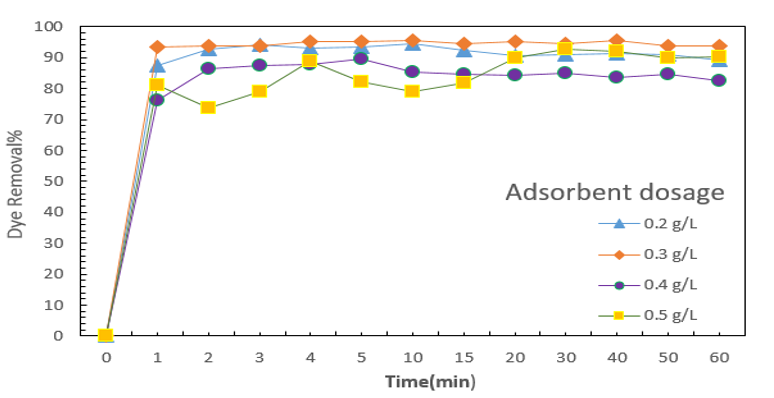
Figure 7: Effect of adsorbent dosage on dye adsorption.
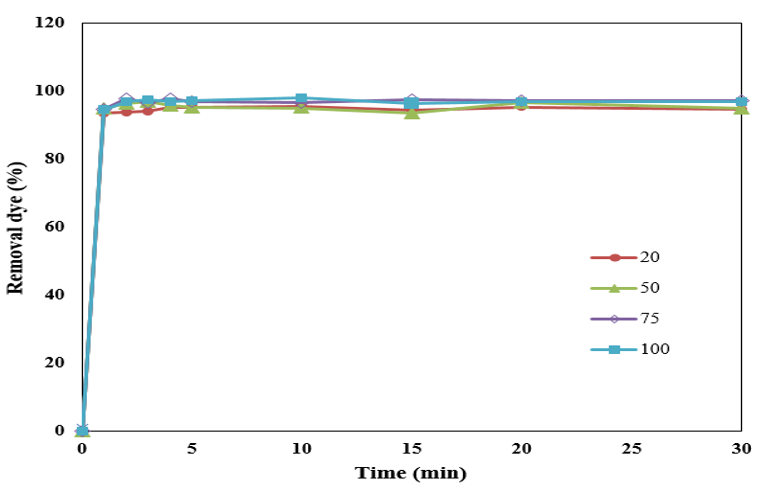
Figure 8: Effect of dye concentration on dye adsorption.

Figure 9: Effect of generation of dendritic structure on dye adsorption.
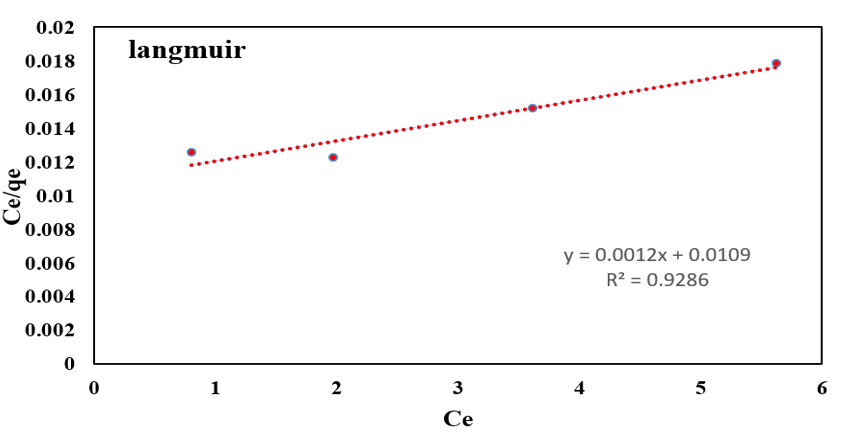
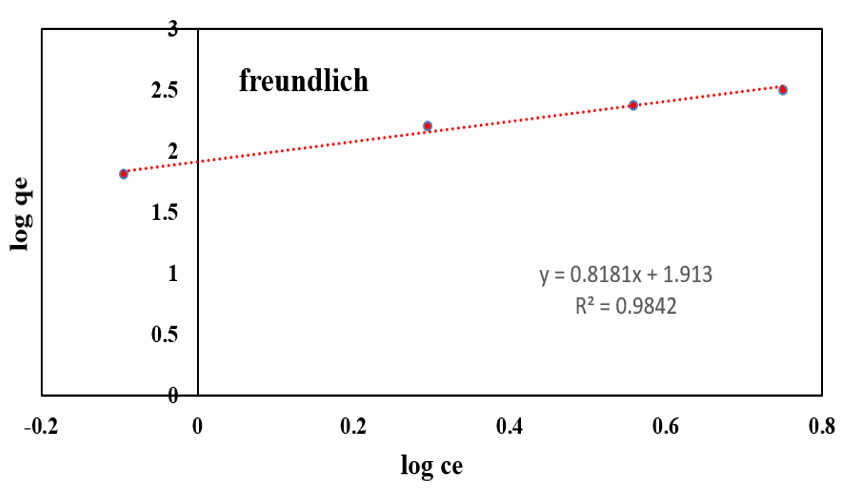
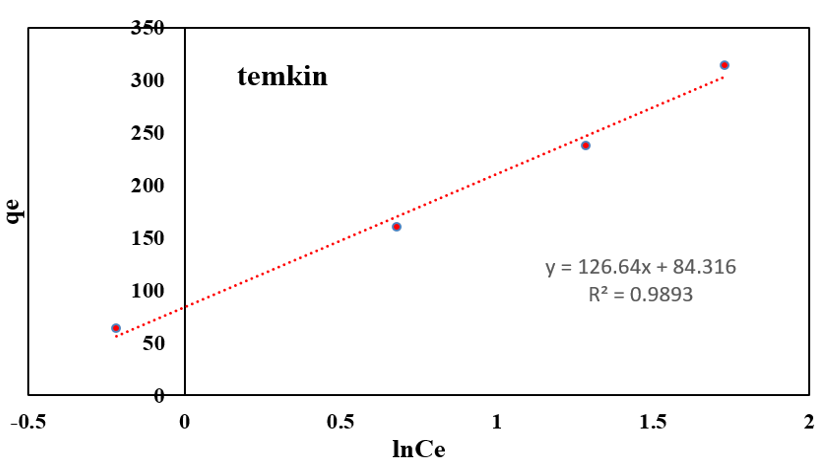
Figure 10: Adsorption isotherm of AR1.
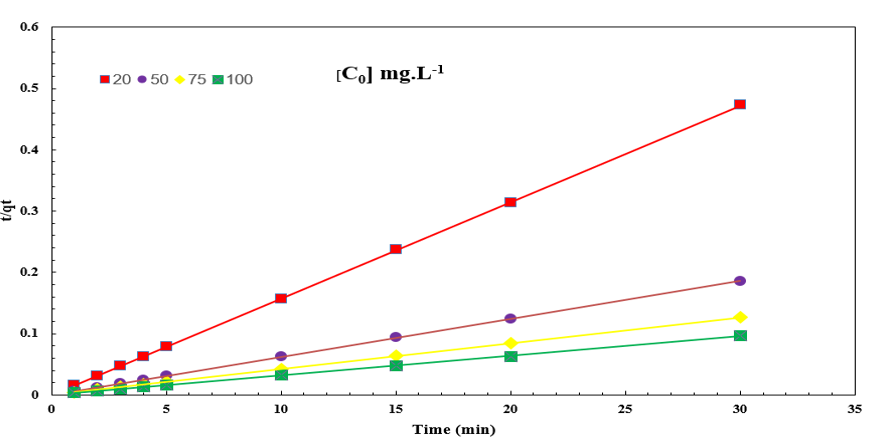
Figure 11: pseudo-second-order adsorption kinetic of AR1.
Conclusion
In this article, amine-terminated dendritic silica was synthesized for functionalization of pristine silica in two divergent and convergent methods. As silica had negative charge due to its surface hydroxyl groups, adsorption of anionic dye may not occur so it required to functionalize this mineral material by amine-terminated dendritic groups to change surface charge. The functionalized adsorption properties confirmed functionalization of this adsorbent. Adsorption capacity of AR1 was enhanced by this functionalization and was showed that it can be efficient adsorbent for removal of AR1. It was reached to maximum dye removal in one minute. Factors like pH, dye concentration and adsorbent dosage were optimized and dye removal percentages in disparate generation was studied. Dye removal and elements percentages of each generation were changed. According to the results, dye removal percentages were enhanced from 0 for pristine silica to 94.8 %for functionalized silica by divergent synthetic route in second generation (G2.0) and 90.6% for functionalized silica by convergent synthetic route in optimum adsorption condition. Although divergent synthetic route was more efficient for adsorption of AR1 in comparison between convergent synthetic routes, convergent route was choosing due to its easier, faster, more uniformity and higher purity synthesis route and it’s clear that dye removal percentages of these routes were almost near to each other.
References
6. Lutwack R, Morrison A (1984) Silicon material preparation and economical watering methods. Material Science and Engineering 75: 215-218.
8. Yang R (2003) Adsorbents: fundamentals and applications. John Wiley & Sons, USA.
12. Yan D, Gao C, Frey H (2011) Hyperbranched polymers: synthesis, properties, and applications. John Wiley & Sons, USA.
14. Zhang Y, Qu R, Sun C, Chen H, Ji C, et al. (2009) Chemical functionalization of silica-gel with diethylenetriamine via an end-group protection approach for adsorption to Hg(II). Applied Surface Science 255(11): 5818-5826
15. De Gennes PG (1983) Wetting: Statics and dynamics. J APS Physics 57: 351-360.
Citation: Goftari S and Akbari S. Comparison between Divergent and Convergent Synthetic Routes of Functionalized Silica by Amine-Terminated Dendritic Materials. J Miner Sci Materials. 2020; 1(1): 1003.